Abstract
Our ability to manage acute myeloid leukemia (AML) is limited by our incomplete understanding of the epigenetic disruption central to leukemogenesis, including improper histone methylation. Here we examine 16 histone H3 genes in 434 primary AML samples and identify Q69H, A26P, R2Q, R8H and K27M/I mutations (1.6%), with higher incidence in secondary AML (9%). These mutations occur in pre-leukemic hematopoietic stem cells (HSCs) and exist in the major leukemic clones in patients. They increase the frequency of functional HSCs, alter differentiation, and amplify leukemic aggressiveness. These effects are dependent on the specific mutation. H3K27 mutation increases the expression of genes involved in erythrocyte and myeloid differentiation with altered H3K27 tri-methylation and K27 acetylation. The functional impact of histone mutations is independent of RUNX1 mutation, although they at times co-occur. This study establishes that H3 mutations are drivers of human pre-cancerous stem cell expansion and important early events in leukemogenesis.
Similar content being viewed by others
Introduction
AML is a heterogeneous type of aggressive leukemia with poor survival, even with the use of intensive cytotoxic treatment1,2. It develops in a progressive manner as pre-leukemic cells accrue mutations leading to the development of full leukemic clones3,4. This can result in a mixed population of leukemic and related pre-leukemic clones in a patient with differing mutational composition3,4. Disrupted epigenetic control occurs during this process, and this is often driven by mutations in epigenetic regulators5,6. However, our incomplete understanding of what drives the altered epigenetic landscape in leukemia and when these factors contribute to disease development and progression impedes our ability to prevent or cure this disease.
Pre-cancerous expansions of mutated hematopoietic clones occur commonly in myeloid disease, such as AML, secondary AML (s-AML), and myelodysplastic syndrome (MDS), and rarely in healthy individuals. These pre-leukemic HSCs represent an intermediate step in leukemogenesis and put individuals at an increased risk for developing de novo or secondary hematological malignancies3,4,7,8,9,10. Mutations found in ASXL1, a regulator of histone methylation, are common alterations found in pre-leukemic HSCs7,8,10. Indeed, post-transcriptional modification of histones, including methylation and acetylation, is commonly deregulated in AML6,11. Mutations in genes associated with the polycomb repressive complex 2 (PRC2) that methylates histone H3 at K27, including EZH2 and ASXL1, are involved in leukemogenesis6,11.
In addition to epigenetic regulators, mutations in histone genes have been previously identified in cancer. Mutations in H3 histone variants at K27 and G34 were first identified in pediatric brain tumors, followed by mutations in H3K36 in sarcomas12,13,14,15,16,17,18. The H3K27 mutations act dominantly to decrease global H3K27 tri-methylation (H3K27me3), at least partially due to inhibition of EZH219. Examination of H3K27 mutations in hematological malignancies have identified them in T-acute lymphoid leukemia (H3.3), myelodysplastic syndrome (H3.3) and AML (H3.1)20,21,22. An initial study in AML suggested that histone mutations occur late in the leukemic progression and are present only in minor clones22. This is unexpected given the key roles of epigenetic modifiers in pre-leukemic development and histone mutations as founding mutations in pediatric brain tumors.
Here we use direct sequencing to examine all histone H3 variants in two cohorts of primary AML and remission samples and observe that histone mutations, including both novel and previously described mutations, can occur in pre-leukemic cells and contribute to the major leukemic clone. Comprehensive in vitro and in vivo analyses reveals that these oncohistones dramatically expand HSC populations and alter differentiation in the myeloid/erythroid lineage. In established human leukemia, H3K27 mutations increase proliferation and aggressive features. The H3 mutations occur at a higher frequency in s-AML. Our results demonstrate a role for oncohistones early in the leukemogenic process and in the expansion of human pre-cancerous stem cells.
Results
H3 histones are mutated in AML with a higher frequency in s-AML
To comprehensively examine the role of histone H3 in AML, we performed targeted MiSeq sequencing of the 16 histone H3 genes for 434 primary human AML samples from two cohorts (Supplementary Tables 1 and 2). We identified seven mutations in variants of histones H3.1 and H3.3 (see Table 1), including at amino acid K27; these were validated by Sanger sequencing (1.6%). The mutations are rare or not detectable in a large dataset of normal samples, suggesting that these are bona-fide somatic mutations in our leukemic samples (gnomAD: frequency of 0.0004% for Q69H, 0.04% for R8H, and not detected for the rest; number of alleles examined 144,258−276,69223). There is significant enrichment for s-AML in H3 mutant samples as three of the seven were found in s-AML (43%) compared to the general frequency of s-AML in our cohorts of 7.6% (Fisher’s exact test: p = 0.011; Tables 1 and 2). Overall, the incidence of all histone mutations in s-AML is 9%. Focusing on K27, where mutation has been shown to be embryonically lethal and confirmed as somatic in other cancers, we observed the same enrichment in s-AML for K27M and K27I (6%, Fisher’s exact test: p = 0.016)22,24,25,26.
H3 mutations exist in pre-leukemic HSCs and major leukemic clones
To determine the clonal representation of histone mutations in AML, we performed exome sequencing of samples from patients 064, 105, and 8760 to identify co-occurring mutations, and we examined our MiSeq and exome data for variant allele frequency (VAF). The VAFs of the histone mutations were consistently high, establishing that the histone mutations were present in the major leukemic clone (Table 1). Furthermore, the high VAF indicated that the histone mutations potentially occurred in pre-leukemic founder clones in our samples. To address this, we examined remission samples from patients 105 and 083 for histone mutations (Table 1). The K27M mutation in the patient 105 diagnosis sample was present with a VAF of 43.4% in the patient’s remission sample. Likewise, in patient 083 the Q69H histone mutation from the diagnosis sample was also detected in the remission sample at a VAF of 54.5%. Further, the t(9;11) translocation present in the primary 083 sample was not detected in the remission sample, demonstrating the acquisition of the Q69H mutation prior to the t(9;11) translocation event. Our data establishes that histone mutations are present in the major leukemic clone in each patient and occur early in leukemogenesis in expanded, pre-leukemic HSCs.
H3 mutations do not exclusively occur in RUNX1-mutated samples
To determine if histone mutations commonly co-occur with mutations in specific genes in our AML samples, we examined the exome sequencing data as well as MiSeq data on IDH1, IDH2, ACVR1, BRAF, and PPM1D (Table 1). In our data, histone mutations did not co-occur exclusively with specific other common mutations. Significantly, a recent study on H3K27 mutations in AML stated that these mutations occur exclusively in samples with RUNX1 alterations, although the sample size was three AML samples22. We determined the RUNX1 status in our samples and observed a RUNX1 mutation in only one of the seven AML samples harboring a histone mutation (Table 1). More specifically, a RUNX1 mutation co-occurred in only one of three K27 mutated samples. Thus, RUNX1 mutations are not obligate co-factors for these histone mutations.
Q69H, K27M, and K27I mutations alter differentiation in vitro
The presence of histone mutations in clonally expanded hematopoietic cells suggests that H3 mutations can alter normal hematopoietic stem or progenitor cell function. To examine this, we performed in vitro colony formation unit (CFU) assays in normal human CD34+ cord blood cells. The H3.1 Q69H mutation distorted differentiation and caused a decrease in myeloid colonies (CFU-GM and CFU-M; Fig. 1a, b). Previously, K27 mutations in H3.3 were shown to lead to an increase in CFU-G/GM colonies, although H3.1 mutations were not examined22. Using a complete cytokine cocktail allowing for detection of a wide range of human progenitor cells, we observed that human CD34+ cord blood cells transduced with H3.1 K27 mutants produce significantly fewer erythroid (BFU-E) and more granulocytic (CFU-G) colonies compared to control cells (Fig. 1c, d).
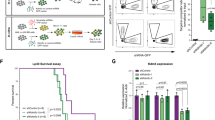
H3.1 mutations alter HSC frequency and hematopoietic differentiation in vitro and in vivo. Number of a granulocyte-macrophage colonies and b macrophage colonies (CFU-M) from CD34+ sorted human cord blood transduced with HIST1H3H WT or Q69H. c, d Number of (c) erythroid (BFU-E) and d granulocytic (CFU-G) colonies from CD34+ sorted human cord blood transduced with HIST1H3H WT/K27M or HIST1H3F WT/K27I. For a–d n = 4 and is representative of three independent experiments. e Circulating haemocytes in WT larvae (control), larvae expressing H3.3WT and H3.3K27M counted by Neubauer haemocytometer (n = 40). Data represent mean ± standard error of the mean. f Design of in vivo xenotransplantation of transduced CB cells injected into sublethally-irradiated NSG mice. g–n Flow cytometry analysis of populations from the bone marrow of the injected femur of mice xenotransplanted with CD34+CD38- human cord blood cells transduced with HIST1H3H WT/K27M or HIST1H3F WT/K27I after 12–14 weeks. Data is representative of two independent experiments. g Frequency of CD34+CD38- HSPCs in the CD45+GFP+ population (n = 6). h Frequency of HSC1 (CD45RA−CD90+CD49f+) and i HSC2 (CD45RA-CD90−CD49f+) in the CD34+CD38− population. j Frequency of CMP (CD135+CD45RA−), k MEP (CD135−CD45RA−) and l GMP (CD135+CD45RA+) in the CD34+CD38+CD7−CD10− population. Data for h–l represents pooled pairs of samples; n = 3. m Frequency of granulocytes (CD33dim, SSChigh) in the CD45+ population and n ratio of CD71+ erythroid cells to CD71- erythroid cells in the CD45−GlyA+ populations (n = 6). o Schematic depicting the changes in frequency of HSCs and the block of differentiation in the erythroid lineage with H3.1 K27M/I mutations. See Supplementary Figs. 1 and 3 for gating strategy used. Data represents mean ± standard deviation. Statistical analysis was performed by two-way Student’s t-tests. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
To confirm the fundamental impact of histone mutations on hematopoiesis, we examined them in a primitive blood system. Drosophila melanogaster that overexpress H3K27M mutated histones have increased circulating haemocytes in larvae compared to wildtype (Fig. 1e). This supports a critical role for histones in activating cellular innate immune response in Drosophila and reflects the central importance of the H3K27 histone mutations.
H3.1 K27M/I drives HSC expansion and alters differentiation in vivo
Next, we assessed the functional effect of histone H3.1 mutations in HSCs, potentially leading to pre-leukemic expansion. A previous study showed that the expression of H3.3 K27 mutants in human CD34+ cells led to an expansion of phenotypically identified HSCs in vitro but a decrease in chimerism in vivo, suggesting that H3.3 K27 mutations could have an impact on HSC function despite the ambiguity of these results22. We performed quantitative in vivo functional HSC assays and used K27 mutants in H3.1 variants, as mutations in H3.3 are rarely found in AML. We transduced human CD34+CD38− cells with lentiviral vectors to express HIST1H3H (K27M or wildtype) or HIST1H3F (K27I or wildtype) and transplanted these into mice (Fig. 1f). There was a substantial increase in the stem cell-enriched population (CD34+CD38−) in the mutant samples compared to controls after 14 weeks (Fig. 1g). Within this population, the K27M mutant drove a large expansion in the most primitive human HSC population (HSC1: CD45RA−CD90+CD49f+) while K27I expanded the HSC2 population (CD45RA−CD90−CD49f+; Fig. 1h, i, see Supplementary Fig. 1 for gating strategy and Supplementary Fig. 2 for complete data). To functionally assess these HSCs, we re-injected the transduced cells (CD45+GFP+) into secondary mice and measured engraftment after 14 weeks. We injected less than one million CD45+ cells, which is expected to contain few HSCs and does not generally result in robust engraftment in secondary mice. The mice injected with K27 mutant cells were highly engrafted (average 30% engraftment; 6/6 mice) while only one control mouse was engrafted (1/6 mice, 3.1% engraftment), confirming that K27 histone mutations expand functional HSCs in long-term assays (Mann–Whitney test: p = 0.004) (Table 3). Thus, histone mutations enlarge the mutant HSC population, consistent with the clonal expansion we observed in patient samples.
We then examined whether the K27 histone mutations altered the in vivo differentiation of hematopoietic cells derived from the HSCs. After 14 weeks in vivo, we observed an expansion of common myeloid progenitors (CMPs) and megakaryocyte-erythroid progenitors (MEPs) and altered frequencies of multiple other populations in the K27M xenotransplant experiments, including granulocyte-monocyte progenitors (GMPs) (Fig. 1j–l and Supplementary Fig. 2 for complete analysis). The K27I mutation affected differentiation to a lesser extent than K27M in keeping with previous observations of the impact of the different amino acid substitutions on histone methylation (Supplementary Fig. 2)19,27. In the mature cells and lineage-committed fractions we detected an increase in granulocytes in the mutants (Fig. 1m, Supplementary Figs. 3 and 4). Moreover, we observed a blockage of differentiation in the erythroid lineage in the mutants with an increase in GlyA+CD71+ cells but a decrease in the downstream GlyA+CD71– cells (Fig. 1n, Supplementary Fig. 4). This is consistent with the CFU results. Thus, hematopoietic cells with histone H3.1 mutants have altered myeloid differentiation with a bias towards the erythroid-megakaryocyte lineage and a blockage downstream in erythroid maturation (Fig. 1o).
H3.1 K27M/I increase human AML proliferation and aggressiveness
Next, to examine the role of oncohistones in established human AML, we examined TEX cells, a line derived from CD34+-enriched human cells transduced with the TLS-ERG oncogene, a fusion gene that occurs in s-AML/MDS28. Transduction with K27M/I mutant H3.1 histones led to a dramatic increase in proliferation compared to controls (Fig. 2a). In addition, these cells showed a 3-fold increase in functional stem/progenitor cells as determined by colony formation assays (Fig. 2b). To assess the impact of oncohistones in vivo, we injected a 1:1 mix of transduced and untransduced cells into the right femur of NSG-S mice and examined the bone marrow and spleens 5 weeks later (Fig. 2c). The TEX cells transduced with mutant histones outcompeted untransduced TEX cells with a 9:1 ratio of transduced to untransduced after 5 weeks while wildtype or Luc2-control transduced cells did not show this competitive advantage (Fig. 2d). Additionally, TEX cells transduced with wildtype histones or Luc2-control displayed an average engraftment of 21% in the injected femur while mutant TEX cells engrafted at >60%, indicating that mutant TEX cells are more successful in outcompeting endogenous mouse bone marrow cells compared to controls (Fig. 2e). Furthermore, mutant TEX cells presented with additional features of a more aggressive disease. They gained the ability to engraft the spleen (Fig. 2f) and had dramatically increased engraftment in the contralateral femur (Fig. 2g). Additionally, only TEX cells transduced with K27M/I mutants, but not wildtype histones or Luc2-control, produced solid masses of leukemic cells at the site of injection (Fig. 2h). Overall, we observed that H3.1 K27M/I mutant histones increase the proliferation and aggressiveness of human leukemic cells.
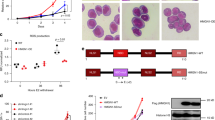
H3.1 K27M/I mutations increase the proliferation of AML cells in vitro and in vivo. a In vitro cell proliferation assay of TEX cells transduced with HIST1H3H WT (blue), HIST1H3H K27M (red), HIST1H3F WT (green), HIST1H3F K27I (pink), and Luc2 control (black) (n = 3, representative of two independent experiments). b Colony formation unit assay of TEX cells transduced with HIST1H3H WT, HIST1H3H K27M, HIST1H3F WT, HIST1H3F K27I, and Luc2 control (n = 4, representative of three independent experiments). c Design of in vivo xenotransplantation of transduced TEX cells mixed equally with untransduced TEX cells into sublethally-irradiated NSG-S mice. d–g Flow cytometry analysis of mice xenotransplanted with TEX cells transduced with HIST1H3H WT/K27M, HIST1H3F WT/K27I, or Luc2 control for 5 weeks. Data is representative of two independent experiments. d Percent GFP+ transduced cells in the human (CD45+) population 5 weeks post-injection of TEX cells. e–g Percent GFP+CD45+ engraftment of e the injected femur, f the spleen, and g the contralateral femur 5 weeks post-injection of TEX cells. h Representative image of injected femur and surrounding tissue of mice intrafemorally injected with TEX cells overexpressing HIST1H3H WT (top panel) or HIST1H3H K27M (bottom panel). Flow cytometry analysis indicates the solid mass consists of TEX cells (95.4% human CD45+ transduced cells) (n = 5). Data represents mean ± standard deviation. Statistical analysis was performed by two-way Student’s t-tests. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
H3.1 K27M/I alter gene expression, H3K27 methylation and acetylation
We next examined the underlying effects of histone mutations on H3K27me3, H3K27 acetylation (H3K27ac), and gene expression. Immunoblotting revealed that TEX cells expressing the K27 mutated H3.1 histones lead to a global decrease in H3K27me3 compared to controls (Fig. 3a), as previously observed22,29. RNA-seq analysis of TEX cells revealed that K27 mutations alter gene expression (Fig. 3b, c; see Supplementary Data 1 for complete RNA-seq and ChIP-seq data). Most of the affected genes showed increased expression in both K27M and K27I cells (Fig. 3b, c). Specifically, we identified 118 genes as significantly upregulated in H3.1 K27M cells compared to only 46 genes in K27I cells (Fig. 3b–d, Supplementary Data 2 for gene lists). ChIP-seq analysis of all annotated promoters revealed a global decrease in H3K27me3 and an inverse correlation with gene expression (Fig. 3b, c, e). Consistent with transcriptome analysis, loss of H3K27me3 was more severe in TEX cells overexpressing K27M compared to the K27I mutation. We specifically examined the H3K27me3 levels at the promoters of the 118 K27M and 46 K27I upregulated genes. In wildtype cells, H3K27me3 enrichment levels at these promoters were higher than the median global promoter levels, while the K27 mutants both showed significant large decreases (Fig. 3d, e, h, i). Finally, we confirmed that the mutant and wildtype histones have a similar pattern of DNA localization in leukemic cells, indicating that the effects we observed are not driven by mutant-specific histone localization (Supplementary Fig. 5 and Supplementary Data 3). These analyses suggest that upregulated genes are direct targets of H3K27me3-mediated repression in wildtype cells, and upon expression of K27M, and to a lesser extent K27I, the reduction in H3K27me3 leads to up-regulation.
H3.1 K27M/I mutations modify H3K27me3 and H3K27ac marks and alter gene expression in leukemic cells. a Western blot analysis of H3K27me3 levels from total histone protein from TEX cells transduced with HIST1H3H WT/K27M, HIST1H3F WT/K27I, Luc2 control, or untransduced (UT) cells. b Volcano plot depicting genes showing differential expression in HIST1H3H K27M relative to WT. H3K27me3 change of K27M/WT is overlaid as a heatmap, with red and blue representing loss and gain, respectively. Dashed gates indicate genes called as being significantly down- or upregulated, using threshold of |z-score| >1.5, p-value <0.05. c Volcano plot depicting genes showing differential expression in HIST1H3F K27I relative to WT, as in b. d Venn diagram showing overlap of significantly upregulated genes observed in K27M and K27I expressing TEX cells. e Box-whisker plot showing change of promoter-specific H3K27me3 in the TEX cells, comparing all annotated promoters and the subset that showed up-regulation in both K27M and K27I. The lower and upper whisker represents the minimum and maximum, respectively, after removing outliers where the upper whisker = min(max(x), Q3+1.5 × IQR) and lower whisker = max(min(x), Q1−1.5 × IQR), where IQR = Q3-Q1. The two ‘hinges’ are versions of the first and third quartile. The notches extend to ± 1.58 IQR/sqrt(n) representing a confidence interval. IQR stands for interquartile range. Center line indicates median. f, g two-dimensional scatterplot depicting the change of H3K27me3 and H3K27ac at all annotated promoters in f K27M and g K27I overexpressing TEX cells. Corresponding K27M/WT RNA-seq z-score is overlaid as a heatmap, with red and blue representing up- and down-regulation, respectively. h, i Genome browser snapshot of the h GATA1 locus and i LIF locus with RNA-seq and Rx-normalized H3K27me3 and H3K27ac data
Next, we examined if these upregulated genes may account for the functional differences seen in K27 mutant cells compared to wildtype cells. Ontology analysis revealed processes involved in hematopoietic differentiation, including general myeloid and erythroid differentiation (Table 4). In order to capture a broad range of differentially expressed genes, we performed a second analysis using a more generous threshold of detection. We identified 360 upregulated and 78 downregulated genes for K27M and 190 upregulated and 52 downregulated genes for K27I (Supplementary Fig 6, Supplementary Data 2). Additional processes identified include immune regulation, chemotaxis and adhesion, with interferon gamma mediated signaling identified specifically in the downregulated genes (Supplementary Fig 6e).
It has been demonstrated that K27 mutations can alter the distribution of the H3K27ac activation mark19,30. Intersection of H3K27ac ChIP-seq results with RNA-seq and H3K27me3 in TEX cells showed that loss of H3K27me3 was not concomitant with H3K27ac gain per se. Rather, the promoters of upregulated genes, such as GATA1 and LIF, have higher H3K27ac (Fig. 3f–i). Thus, global loss of the repressive H3K27me3 in H3.1 K27 mutants results in ectopic gain of H3K27ac and up-regulation of hematopoietic genes.
One of the few genes whose expression was significantly decreased in TEX cells transduced with K27 mutant H3.1 histones is CDKN2A, suggesting that histone mutations drive proliferation through cell division (Supplementary Data 1). Suppressed expression of CDKN2A has been observed in glioma cells with H3K27 mutations and rescued with EZH2 inhibitors31,32. However, we observed a protective role for the H3.1 K27 mutations against treatment with the EZH2 inhibitor UNC1999 (Supplementary Fig. 7). In addition, we show that H3.1 K27M AML cells are not more sensitive to drugs targeting other epigenetic regulators such as inhibitors against the histone demethylase JMJD3 (GSK-J4), BRD4 (JQ1) and histone deacetylases (vorinostat, trichostatin A and panobinostat) (Supplementary Fig. 7). Overall, the functional alterations driven by H3.1 K27M epigenetic modifications are robust and difficult to treat with such therapies.
Discussion
An early step in the leukemogenic process is the alteration of epigenetic regulators, resulting in a disrupted epigenetic landscape in leukemic cells. The mutations involved and their effects are complex and not fully understood. The well-established role of histone modifications in leukemia led us to examine two cohorts of AML samples in which we identified mutations in histone H3 variants. These mutations expand functional HSCs (K27M/I), alter differentiation (K27M/I and Q69H), and lead to an increase in proliferation and other indicators of aggressiveness in leukemia (K27M/I). This represents a fundamental new mechanism for the epigenetic alterations driving leukemogenesis.
We observed a higher frequency of H3 mutations in s-AML. Mutations involved with PRCs such as ASXL1, EZH2, and BCOR are linked to s-AMLs and their antecedent MDS33,34,35. A mutation in these genes is over 95% specific for s-AML33. Thus, the enrichment of histone mutations in s-AML in our cohorts is consistent with the altered regulation of histone methylation seen in s-AML/MDS. In further support of a role for histone mutations in s-AML and prior MDS, an H3F3A K27N mutation with unknown function has been previously observed in an MDS sample21.
Substantial clonal expansion of the normal bone marrow, resulting in high VAFs, has been observed in AML patients, and in s-AML patients in particular5,36,37. High VAFs have been found in leukemic samples but also VAFs of approximately 50% have been observed for known oncogenic driver mutations in bone marrow cells in remission samples. In the case of MDS and s-AML nearly all the bone marrow cells are clonal, even though by definition malignant cells are fewer than 20% in MDS patients36. Thus, histone mutations may act as other oncogenic drivers in myeloid malignancies and contribute to the almost complete clonal dominance of pre-leukemic cells over wildtype cells in the bone marrow.
Unlike a recent study, we did not find that the impact of histone mutations is reliant on mutated RUNX122. Only one of seven AML samples with a histone mutation in our cohorts had a corresponding RUNX1 alteration. Furthermore, histone mutations trigger substantial functional changes in leukemic TEX cells and normal cord blood cells, both of which are wildtype for RUNX1 and the AML1/ETO fusion. As discussed above, s-AML represents a subtype of AML with specific associated mutations33. This includes RUNX1 and mutations in this gene have been shown to co-occur with mutations in ASXL1 and EZH233,38,39. The high frequency of RUNX1 mutations in s-AML may account for the previous observation of co-occurrence of histone H3K27 mutations with RUNX1 alterations22.
In one sample in our cohort we observed both an ASXL1 and HIST1H3F K27I mutation. However, ASXL1 mutations can occur with EZH2 mutations in myeloid malignancies33,40,41, suggesting that ASXL1 mutations do not phenocopy histone H3.1 or EZH2 mutations. This highlights the complexity of epigenome regulation in cancer as the co-occurrence of mutations in genes within shared pathways indicates that they have unique roles.
Histone mutations may serve as potential therapeutic targets in AML patients, either by treating leukemic cells or pre-leukemic clones. Gliomas harboring H3K27M mutations have been shown to be more sensitive to EZH2 inhibitors than wildtype H3 gliomas. This effect is thought to be mediated by the de-repression of CDKN2A31. Despite also seeing a decrease in CDKN2A expression in AML cells expressing H3.1 K27M/I, we did not observe increased sensitivity to EZH2 inhibition. In addition, mutant cells are not more sensitive to drugs targeting components of additional epigenetic processes such as JMDJ3, BRD4 and HDACs. Therefore, these compounds may not be appropriate for H3K27 mutant myeloid malignancies and other therapeutics that can modulate the epigenome may be necessary.
Overall, our data establishes that histone mutations can drive pre-leukemic HSC expansion, with concurrent altered myeloid/erythroid lineage differentiation, and contribute to leukemic aggressiveness. Our data is evidence of an oncohistone-driven expansion of pre-neoplastic primary human stem cells and we speculate that this occurs in cancer types beyond leukemia.
Methods
Sample characteristics and pathological review
Two cohorts were included in the study. The first consisted of 122 patients diagnosed with AML at the American University of Beirut Medical Center (AUBMC), and the second one included 312 patients diagnosed with AML at the University Health Network, Toronto. The study was approved by the institutional review board at both centers (UHN; REB# 01-0573-C), and a shared database with strictly defined variables was created to avoid bias. All subjects involved in the study provided written, informed consent.
Bone marrow samples at AUBMC were obtained from AML patients who had their DNA previously collected for routine diagnostic purposes and stored in the Department of Pathology and Laboratory Medicine as part of the College of American pathologist accreditation requirements from January 2005 to January 2015. Karyotypic analysis (Supplementary Table 1) “Other” category includes+8, −7, -Y,+11,+13,+15,+19,+21,+22, and alterations such as rare translocations and rare deletions.
MiSeq sequencing
We performed deep targeted sequencing on DNA from 442 AML samples from Lebanon (N = 122) and Toronto (N = 312) using an Illumina MiSeq platform (McGill University). The MiSeq panel covers exon 2 of H3.3 (3 H3.3 genes), coding regions of H3.1 and H3.2 isoforms (10 H3.1 and 3 H3.2 genes) as well as mutation hotspots in IDH1 (codon 132), IDH2 (codons 140 and 172), ACVR1 (exons 6–9), BRAF (V600E) and PPM1D (exon 6). The sequencing data were analyzed as previously described13,42. We achieved median coverage of 7000 and 16,000 reads per region in the Lebanese and Toronto cohorts, respectively. Only those variants with allele frequency ≥10% and that were supported by at least 20 alternate reads were retained. These variants were then filtered for common SNPs found in public databases such as the 1000 Genomes Project, dbSNP, and the NHLBI Exome Variant Server (ESP). Sanger sequencing was performed on the DNMT3A gene with the full coding regions of the gene being sequenced in the 122 AML samples from Lebanon.
Whole exome sequencing
Exomes were captured using the Agilent SureSelect All Exon kit v5 kit according to the manufacturer’s instructions and described previously24. Exome sequencing was performed on samples from patients 064, 105, and 8760, including the remission sample for 105. Exome sequencing was performed on samples identified with a H3K27 mutation to identify any co-occurring mutations. The enriched libraries were sequenced on either the Illumina HiSeq 2500 or HiSeq 4000 with 100 bp paired-end reads. Sequence reads were mapped to the human reference genome (hg19) with Burrows-Wheeler Aligner (BWA)43, and duplicate reads were flagged using Picard (http://picard.sourceforge.net) and excluded from further analyses. Variants were called using three different variant callers including SAMtools Mpileup44, FreeBayes version v1.1.0-4-gb6041c6 (Haplotype-based variant detection from short-read sequencing. E Garrison, G Marth - arXiv:1207.3907, 2012), and GATK haplotype caller version 3.845, and were filtered to require at least 20% of reads supporting the variant call. In order to keep the high confidence variant calls, we only kept those variants that were called by at least two of three variant callers. Mutations were annotated using both ANNOVAR46 and custom scripts. Annotated variants were filtered against the common germline polymorphisms present in dbSNP135, the 1000 Genomes project47, NHLBI GO Exomes and an in-house database of approximately 3000 exomes previously sequenced at our center.
Sanger sequencing of RUNX1
We performed Sanger sequencing of exons 3–8 of the RUNX1 gene in AML samples from patients 073, 083, 095, 0561 and the remission sample from 083. The primer sequences are as follows:
RUNX1 exon 3 forward; GCTGTTTGCAGGGTCCTAA, reverse; CCTGTCCTCCCACCACCCTC. RUNX1 exon 4 forward; CATTGCTATTCCTCTGCAACC, reverse; TGCCATGAAACGTGTTTCAAGC.
RUNX1 exon 5 forward; TCAGGCCACCAACCTCATTCTG, reverse; CCAGCCCCAAGTGGATGCAC. RUNX1 exon 6 forward; AGCCCCAGTTTTAGGAAATCCAC, reverse; AGCATCAAGGGGAAACCCC. RUNX1 exon 7 forward; CCCACCCCACTTTACATATAATTG, reverse; CCAGCTCAGCTGCAAAGAATGTG. RUNX1 exon 8 forward; CCGCAACCTCCTACTCACTT, reverse; GCTTGTCGCGAACAGGAG48.
Collection of cord blood and cell culture
Human cord blood was obtained from full-term deliveries from healthy donors according to the procedures approved by the Institutional Review Boards of Héma-Québec, Sainte-Justine’s Hospital, University of Montreal and the McGill University Health Centre. Written informed consent was given by women during pregnancy within the province of Québec. Mononuclear cells were obtained by centrifugation on Ficoll (GE Healthcare) and were enriched for CD34+ cells using the EasySep CD34+ positive selection kit (STEMCELL Technologies) as per the manufacturer’s protocol. CD34+ enriched human cord blood was cultured in StemSpan SFEM II (STEMCELL Technologies) containing penicillin/streptomycin (Life Technologies), 10 ng/mL IL-6, 100 ng/mL SCF, 100 ng/mL FLT3L, 10ng/mL G-CSF, and 15 ng/mL TPO (Life Technologies).
TEX human leukemia cells (a gift from Dr. J. Dick, Toronto, Canada) were cultured in IMDM (Hyclone) containing 4mM l-glutamine (Life Technologies), 15% fetal bovine serum (FBS) (Wisent), penicillin/streptomycin (Life Technologies), 20 ng/ml SCF and 2 ng/mL IL-3 (Life Technologies). 293FT cells were cultured in high-glucose DMEM (Life Technologies) containing 10% Cosmic Calf Serum (CCS) (GE Healthcare Life Sciences), 0.1 mM MEM non-essential amino acids (Wisent), 6mM L-glutamine, 1Mm MEM sodium pyruvate (Life Technologies), penicillin/streptomycin and 500 μg/mL G-418 (Wisent). Cells were incubated at 37 °C with 5% CO2. Cells were routinely checked for mycoplasma contamination using the Mycoalert Detection kit (Lonza) and were negative.
Lentiviral vector production
The previously described pSMAL vector modified from the MA1 lentiviral vector to have a Gateway cassette and SFFV promoter was used as the backbone for all generated lentiviral vectors49,50. HIST1H3H WT/K27M and HIST1H3F WT/K27I with a C-terminal HA-tag and attB sites for Gateway cloning were generated using GeneArt™ DNA Strings Fragments (Life Technologies). Luciferase modified for human expression (Luc2) was amplified out of the pGL4.51[luc2/CMV/NEO] vector (Promega). Genes were cloned into the pDONR221 vector (Invitrogen) using BP Clonase (Invitrogen) and subsequently cloned into the pSMAL vector using LR Clonase (Invitrogen) as per the manufacturer’s instructions. Lentiviral particles were produced in 293FT cells (Life Technologies) as previously. Viral titer was determined by adding serial dilutions of the concentrated viral particles onto 8227 AML cells and the percentage of GFP+ cells was measured 96 h later by flow cytometry.
Lentivirus transduction
pSMAL lentiviral vectors were used to transduce TEX and cord blood cells at an MOI of 3–549,51. After 16 h, additional appropriate media was added and transduction efficiency was measured using flow cytometry for GFP after 96 h. In transduced TEX cells the K27I mutation accounts for 26% of the HIST1H3F reads and 4% of the total H3 reads with the K27M mutation at 20% of the HIST1H3H reads and 3.6% of the total H3 expression.
Fluorescence-activated cell sorting and flow cytometry
To isolate transduced cells and identify specific populations of cord blood, cells were stained with desired surface markers in PBS with 2% cosmic calf serum (CCS) (Hyclone) and sorted using a BD FACSAria. Flow cytometry was performed using a BD LSRFortessa. Antibodies used are described below and in the supplementary methods. All antibodies were obtained from Biolegend and BD Biosciences. SYTOX Blue (Life Technologies) was used to distinguish live cells from dead cells.
Antibody details
CD34 (APC, APC-Cy7; clone 581), CD38 (PE; clone HB-7), CD45 (AlexaFluor 700; clone 2D1), CD7 (Pe-Cy7; clone CD7-6B7), CD10 (Pe-Cy5; clone HI10a), CD45RA (BV650; clone HI100), FLT3 (PerCP-Cy5.5; clone BV10A4H2), CD90 (BV605; clone 5E10), CD49f (APC; clone GoH3), CD235a (PerCP-Cy5.5; clone HI264), CD71 (PE; clone MA712), CD41 (Pe-Cy7; clone HIP8), CD14 (PE-Dazzle; clone HCD14), CD33 (APC; clone WM53), CD3 (BV605; clone SK7), CD19 (PerCP-Cy5.5; clone HIB19), and CD56 (PE-Cy5; clone hCD56). All antibodies were obtained from Biolegend and BD Biosciences.
Growth and methylcellulose colony-forming unit assays
Transduced TEX cells were cultured in the conditions described in the supplementary methods and harvested every 3 days, counted and reseeded. Cumulative growth was calculated over 21 days. Details for measuring viability after drug exposure are defined in the supplementary methods. Enriched methylcellulose (H4435 STEMCELL Technologies) was used for all assays. 2,000 TEX cells were plated into methylcellulose and colonies were counted 10 days later. 500 transduced cord blood cells sorted for GFP+CD34+ were plated into methylcellulose and colonies were classified and counted 14 days later. Colony types were identified by morphology and color, as per standard criteria.
Drosophila stocks and generation of H3.3 WT and H3.3 K27M transgenic flies
Transgenic flies harboring human H3.3 WT and K27M histones were generated using the Phi C31 integrase system. A pUASg.attB Gateway vector containing the human cDNA with 1xHA (C-terminus) was injected into the ZH-attP line on the third chromosome zh-86Fb (3R 86F) for GAL4-UAS expression. A hemocyte-specific driver (Cg-GAL4.A) (#7011) was obtained from BDSC52. Flies were crossed at 25 °C. White W1118 flies were used as a wildtype control.
Haemocyte counting
H3.3 WT and K27M flies were crossed to the Cg-Gal4 haemocyte driver. Third instar larvae of the indicated genotypes were bled into PBS (SIGMA) and haemocytes were counted. Forty larvae of each genotype (three independent crosses) were counted in each experiment and statistical analysis was performed using Student’s t-tests P < 0.05 (n = 40).
Xenotransplantations
Mouse xenografts were performed according to protocols approved by McGill University and its affiliated Hospitals’ Research Institutes.
TEX cells xenotransplant
NOD-scid IL2Rgnull-3/GM/SF (NSG-S) mice were irradiated with 2.1 Gy using a X-RAD SmART Irradiator (Precision X-Ray, Inc) 24 h prior to intrafemoral injection of 2 million TEX cells (1:1 mixture of untransduced to transduced). Mice were sacrificed 5 weeks after injection (onset of illness) and cells from the injected femur, contralateral femur and spleen were collected for flow cytometry. Human engraftment (CD45+) and GFP percentage were evaluated by flow cytometry for all collected tissue.
Cord blood xenotransplant
CD34+ enriched cord blood was fluorescence-activated sorted for CD34+CD38−. 10,000–20,000 cells per mouse were transduced with lentiviral vectors (MOI 3–5) and maintained in culture for 4 days. NOD-scid IL2Rgammanull (NSG) mice were irradiated with 2.1 Gy 24 h prior and injected intrafemorally with the transduced cord blood cells. Mice were sacrificed 12–14 weeks after injection and bone marrow from the injected femur, contralateral femur and spleen were collected for flow cytometry analysis. Flow cytometry was done to evaluate human engraftment (human-specific CD45 and GFP) and hematopoietic differentiation (CD34, CD38, CD235a (GlyA), CD33, CD19, CD56, CD3, CD41, CD71, and CD14). Bone marrow cells from the injected femur were mouse cell-depleted (STEMCELL Technologies 19849) and lineage-depleted using EasySepTM (STEMCELL Technologies 19356) as per the manufacturer’s protocol prior to analysis of primitive populations by flow cytometry using antibodies against CD34, CD38, CD7, CD10, CD45RA, FLT3, CD90, and CD49f.
Secondary transplantation
GFP+CD45+ cells were sorted from pooled bone marrow from the right and left femurs of primary mice. 852,500 to 975,000 cells were injected intrafemorally into sublethally-irradiated NSG mice. Mice were sacrificed 14 weeks after injection and bone marrow from the injected femur, contralateral femur and spleen were collected for flow cytometry analysis. Flow cytometry was done to evaluate human engraftment (hCD45 and GFP).
Immunoblotting
Total histones were extracted, run on SDS-PAGE and immunoblotted by conventional methods and described elsewhere24. Antibodies used were anti-H3K27me3 (1:2500; Millipore 07–449), anti-Total H3 (1:5000; ab1791), anti-HA (1:2500; CST 3724), and HRP-conjugated anti-rabbit IgG secondary antibody (1:5000, NA934V). Quantification of the ratios of H3K27me3/H3 total were calculated using the Image Lab 5.2.1 software. Uncropped immunoblots can be found in the Source Data.
ChIP and RNA-sequencing on TEX cells
ChIP-seq and RNA-seq preparation and sequencing were carried out as described previously24. Cells were fixed with 1% formaldehyde (Sigma). Fixed cell preparations were washed, pelleted and stored at −80 °C. Sonication of lysed nuclei (lysed in a buffer containing 1% SDS) was performed on a BioRuptor UCD-300 for 60 cycles (10 s on 20 s off), centrifuged every 15 cycles, and chilled by 4 °C water cooler. Samples were checked for sonication efficiency using the criteria of 150–500 bp by gel electrophoresis. After sonication, the chromatin was diluted to reduce SDS level to 0.1% and before the ChIP reaction 2% of sonicated Drosophila S2 cell chromatin was spiked-in the samples for quantification of total levels of histone mark after the sequencing.
ChIP reaction was performed on a Diagenode SX-8G IP-Star Compact using Diagenode automated Ideal ChIP-seq Kit. For H3K27me3 and HA-Tag, 80 μl magnetic beads (Dynabeads M-280 Sheep Anti-Mouse IgG) were washed and then incubated with 2 μg of H3K27me3 antibody (Active Motif, #61017) and 2 million cells of sonicated cell lysate combined with protease inhibitors for 10 h, followed by 20 min wash cycle with provided wash buffers. For H3K27ac, 30 μl magnetic beads (Dynabeads Protein A) were washed and then incubated with 5 μg of H3K27ac antibody (Diagenode, C15410196). For HA-tag, the HA-tag antibody (Cell Signaling Technologies, 3724, rabbit monoclonal) was used at a 1:100 dilution. Reverse cross linking took place on a heat block at 65 °C for 4 h. ChIP samples were then treated with 2 μl RNase Cocktail at 65 °C for 30 min followed by 2 μl Proteinase K at 65 °C for 30 min. Then, samples were purified with QIAGEN MiniElute PCR purification kit as per manufacturers’ protocol. In parallel, input samples were reverse crosslinked and DNA was isolated following the same protocol. Library preparation was carried out using Kapa HTP Illumina library preparation reagents. In brief, 25 μl of ChIP sample was incubated with 45 μl end repair mix at 20 °C for 30 min followed by Ampure XP bead purification. A tailing: bead-bound sample was incubated with 50 μl buffer enzyme mix for 30 min at 30 °C, followed by PEG/NaCl purification. Adapter ligation: bead-bound sample was incubated with 45 μl buffer enzyme mix and 5 μl of different TruSeq DNA adapters (Illumina) for each sample, for 15 min at 20 °C, followed by PEG/NaCl purification (twice). Library enrichment: 12 cycles of PCR amplification. Size selection was performed after PCR using a 0.6×/0.8x ratio of Ampure XP beads (double size selection) set to collect 250–450 bp fragments. ChIP libraries were sequenced using Illumina HiSeq 2000 at 50 bp SE reads.
ChIP and RNA-sequencing analysis
Single-end 50 bp ChIP-seq datasets were aligned using bwa-mem (version 0.7.15, default parameters) to 1 K genome hg19. Reads with identical start and end coordinates were discarded as PCR duplicates. Reads were then filtered for mapping quality of >5 and extended by 250 bp. Raw TSS-specific H3K27me3 RPKM calculations were then performed using SeqMonk using ENSEMBL gene annotation. TSS value was calculated in 3 kb centered bins on the transcription start site. H3K27me3 and H3K27ac RPKM values were then manually normalized to spike-in Rx values. The two technical replicates were then averaged. Non-specific enrichment in the input libraries were subtracted from the normalized ChIP RPKM values. Z-score was calculated from the mean RPKM as z = (Mutant–WT)/SQRT(|Mutant|+|WT|).
Total RNA was extracted from cell pellets and mouse tumors using the RNeasy mini kit (Qiagen) according to instructions from the manufacturer. Library preparation was performed with ribosomal RNA (rRNA) depletion according to instructions from the manufacturer (Epicentre) to achieve greater coverage of mRNA and other long non-coding transcripts. Paired-end sequencing was performed on the Illumina HiSeq 2500 platform. RNA-sequencing reads were stripped of adapter sequences using Trimmomatic (v0.32)53. Low-quality bases in the first four positions as well as at the end of each read were removed using a 4-bp sliding window average trim (average phred33 <30). An additional 3 bp was clipped from the start and end of a read if still found of low quality. Short reads (<30 bp) produced as a result of the trimming process were subsequently discarded. The remaining reads were aligned to the hg19 (GRCh37) build of the human genome using STAR (v2.3.0e)54 with default parameters. Reads with identical start and end coordinates were discarded as PCR duplicates. Reads were then filtered for mapping quality of >5. Gene expression RPKM were calculated using only strand-specific reads using SeqMonk using the ENSEMBL gene annotation. Triplicate RNA-seq were averaged prior to calculation of z-score. Z-score was calculated from the mean RPKM as z = (Mutant–WT)/SQRT(Mutant+WT). P-value was generated from a two-tailed t-test. Significant deregulation threshold for RNA-seq: |z| >1.5, p-value <0.05. Expanded analysis using a significant deregulation threshold of |z| >0.8, p-value <0.05. Significantly enriched Gene Ontology were called using PANTHER.
Drug treatment
TEX cells were treated with either DMSO, UNC1999, GSK-J4, JQ1, panobinostat, vorinostat and trichostatin A at indicated concentrations. Drugs were obtained from Tocris and through the Structural Genomic Consortium. Cells were harvested and live cells (SYTOX®-negative) were counted using a BD LSRFortessa with an HTS (BD Biosciences).
Statistical analysis
Unless otherwise stated, mean ± standard deviation values are given and p-values were calculated by two-tailed unpaired Student’s t-test. In the case of secondary transplantation, a Mann–Whitney test was used to compare engraftment between mice injected with wildtype or mutant cells. Boxplots were drawn using R. The center line is the median, the whiskers are minimum and maximum after removing outliers, the box limits are quartiles, and notches are ±1.58 IQR/sqrt(n) and generally represent a 95% confidence interval of median.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Raw exome and transcriptome sequence data have been deposited at the European Genome-phenome Archive (EGA), which is hosted by the European Bioinformatics Institute (EMBLEBI) and the Centre for Genomic Regulation (CRG), under accession number EGAS00001003288 All associated ChIP- and RNA-seq data have been deposited to GEO under the GEO accession GSE122273.
References
Dohner, H. et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129, 424–447 (2017).
Lowenberg, B. et al. Cytarabine dose for acute myeloid leukemia. N. Engl. J. Med. 364, 1027–1036 (2011).
Jan, M. et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci. Transl. Med. 4, 149ra118 (2012).
Shlush, L. I. et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506, 328–333 (2014).
Corces-Zimmerman, M. R., Hong, W.-J., Weissman, I. L., Medeiros, B. C. & Majeti, R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc. Natl Acad. Sci. USA 111, 2548–2553 (2014).
Ntziachristos, P., Abdel-Wahab, O. & Aifantis, I. Emerging concepts of epigenetic dysregulation in hematological malignancies. Nat. Immunol. 17, 1016–1024 (2016).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Jan, M., Ebert, B. L. & Jaiswal, S. Clonal hematopoiesis. Semin. Hematol. 54, 43–50 (2017).
Xie, M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014).
Eriksson, A., Lennartsson, A. & Lehmann, S. Epigenetic aberrations in acute myeloid leukemia: early key events during leukemogenesis. Exp. Hematol. 43, 609–624 (2015).
Behjati, S. et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 45, 1479–1482 (2013).
Fontebasso, A. M. et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat. Genet. 46, 462–466 (2014).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Sturm, D. et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22, 425–437 (2012).
Khuong-Quang, D.-A. et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 124, 439–447 (2012).
Lu, C. et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science 352, 844–849 (2016).
Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44, 251–253 (2012).
Lewis, P. W. et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340, 857–861 (2013).
Atak, Z. K. et al. Comprehensive analysis of transcriptome variation uncovers known and novel driver events in T-cell acute lymphoblastic leukemia. PLoS Genet. 9, e1003997 (2013).
Attieh, Y. et al. Low frequency of H3.3 mutations and upregulated DAXX expression in MDS. Blood 121, 4009–4011 (2013).
Lehnertz, B. et al. H3K27M/I mutations promote context-dependent transformation in acute myeloid leukemia with RUNX1 alterations. Blood 130, 2204–2214 (2017).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Pathania, M. et al. H3.3K27M cooperates with Trp53 loss and PDGFRA gain in mouse embryonic neural progenitor cells to induce invasive high-grade gliomas. Cancer Cell 32, 684–700 (2017). e689.
Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44, 251–253 (2012).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Jayaram, H. et al. S-adenosyl methionine is necessary for inhibition of the methyltransferase G9a by the lysine 9 to methionine mutation on histone H3. Proc. Natl Acad. Sci. USA 113, 6182–6187 (2016).
Warner, J. K. et al. Direct evidence for cooperating genetic events in the leukemic transformation of normal human hematopoietic cells. Leukemia 19, 1794–1805 (2005).
Bender, S. et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24, 660–672 (2013).
Herz, H. M. et al. Histone H3 lysine-to-methionine mutants as a paradigm to study chromatin signaling. Science 345, 1065–1070 (2014).
Mohammad, F. et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat. Med. 23, 483–492 (2017).
Cordero, F. J. et al. Histone H3.3K27M represses p16 to accelerate gliomagenesis in a murine model of DIPG. Mol. Cancer Res. 15, 1243–1254 (2017).
Lindsley, R. C. et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125, 1367–1376 (2015).
Metzeler, K. H. et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 128, 686–698 (2016).
Herviou, L., Cavalli, G., Cartron, G., Klein, B. & Moreaux, J. EZH2 in normal hematopoiesis and hematological malignancies. Oncotarget 7, 2284–2296 (2016).
Walter, M. J. et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 366, 1090–1098 (2012).
Shlush L. I., et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 547, 104–108 (2017).
Gaidzik, V. I. et al. RUNX1 mutations in acute myeloid leukemia are associated with distinct clinico-pathologic and genetic features. Leukemia 30, 2160–2168 (2016).
Khan M., et al. Clinical outcomes and co-occurring mutations in patients with RUNX1-mutated acute myeloid leukemia. Int. J. Mol. Sci. 18, e1618 (2017).
Abdel-Wahab, O. et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia 25, 1200–1202 (2011).
Patnaik, M. M. et al. EZH2 mutations in chronic myelomonocytic leukemia cluster with ASXL1 mutations and their co-occurrence is prognostically detrimental. Blood Cancer J. 8, 12 (2018).
Nikbakht, H. et al. Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nat. Commun. 7, 11185 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Abecasis, G. R. et al. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Fernandez-Mercado, M. et al. Mutation patterns of 16 genes in primary and secondary acute myeloid leukemia (AML) with normal cytogenetics. PLoS ONE 7, e42334 (2012).
Amendola, M., Venneri, M. A., Biffi, A., Vigna, E. & Naldini, L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat. Biotechnol. 23, 108–116 (2005).
van Galen, P. et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature 510, 268–272 (2014).
van Galen, P. et al. Reduced lymphoid lineage priming promotes human hematopoietic stem cell expansion. Cell Stem Cell 14, 94–106 (2014).
Berlandi, J. et al. Identification of genes functionally involved in the detrimental effects of mutant histone H3.3-K27M in Drosophila melanogaster. Neuro-Oncology 21, 628–639 (2019).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA−seq aligner. Bioinformatics 29, 15–21 (2013).
Acknowledgements
This work was in part supported by grants from the Cole Foundation (K.E.), the Canada Research Chair program (K.E.), the Canada Foundation for Innovation (K.E.), The American University of Beirut Medical Center Internal Academic Grant and BMT program (A.B.), The Montreal Children’s Hospital Foundation (K.E.), The American University of Beirut Medical Practice Plan (M.S.), the Orsino Chair in Leukemia Research (M.D.M.), the Wendy, and Elliot Eisen fund (M.D.M.) and fellowships from CIHR (M.B., T.G.), FRQS (M.B., A.S.H., H.M.D., J.P.) and the Cole Foundation (M.B., H.M.D.). N.J. is the recipient of a Chercheur Boursier, Chaire de Recherche Award from the FRQS and holds a US National Institutes of Health (NIH) grant (P01-CA196539). We would like to thank the NHLBI GO Exome Sequencing Project and its ongoing studies which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010). We thank Paul Lasko and Amel Chaouch for kindly sharing the H3.3 WT and H3.3 K27M transgenic flies. We thank the Immunophenotyping platform at the Research Institute of the McGill University Health Centre for all of the cell sorting performed and the Cord Blood Research Bank, lead by Dr. Michel Duval (CHU Sainte-Justine), in partnership with Héma-Québec for providing cord blood samples. We thank John E. Dick for thoughtful discussion of this work.
Author information
Authors and Affiliations
Contributions
M.B., M.S., T.G., N.J., A.B., and K.E. conceived the study and designed the experiments. T.G., L.G.M., and H.N. co-ordinated and analyzed DNA sequencing. M.B., M.S., P.A.T., M.Z., Y.Z., and K.R. performed in vitro assays. M.S. and F.F. performed Drosophila experiments. M.B., K.E., H.M.D., and A.L.N. conducted in vivo xenotransplant experiments. C.C.L.C., N.D.J., and C.L.K. analyzed RNA-seq data. A.S.H. and C.C.L.C. analyzed ChIP-seq data. C.M., R.M., R.N., J.E.C., M.E.S., R.M., M.D.M., and A.B. provided patient samples and clinical data. M.B. and K.E. prepared the manuscript with assistance from M.S., T.G., N.J., and A.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Communications thanks Katia Georgopoulos and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boileau, M., Shirinian, M., Gayden, T. et al. Mutant H3 histones drive human pre-leukemic hematopoietic stem cell expansion and promote leukemic aggressiveness. Nat Commun 10, 2891 (2019). https://doi.org/10.1038/s41467-019-10705-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-10705-z
This article is cited by
-
Oncohistones and disrupted development in pediatric-type diffuse high-grade glioma
Cancer and Metastasis Reviews (2023)
-
Histone 3.3-related chromatinopathy: missense variants throughout H3-3A and H3-3B cause a range of functional consequences across species
Human Genetics (2023)
-
H3.3 safeguards haematopoietic ERV-quilibrium
Nature Cell Biology (2022)
-
Relapse after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia: an overview of prevention and treatment
International Journal of Hematology (2022)
-
Oncohistone interactome profiling uncovers contrasting oncogenic mechanisms and identifies potential therapeutic targets in high grade glioma
Acta Neuropathologica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.