Abstract
Facilitation occurs when one species positively impacts the fitness of another, and has predominantly been studied in free-living species like plants. Facilitation can also occur among symbiont (mutualistic or parasitic) species or strains, but equivalent studies are scarce. To advance an integrated view of the effect of facilitation on symbiont ecology and evolution, we review empirical evidence and their underlying mechanisms, explore the factors favouring its emergence, and discuss its consequences for virulence and transmission. We argue that the facilitation concept can improve understanding of the evolutionary forces shaping symbiont communities and their effects on hosts.
Similar content being viewed by others
Introduction
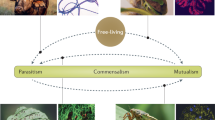
It is now widely accepted that interacting species can positively impact one another1,2,3,4,5. Facilitation (Box 1) is one of the broadest terms referring to these positive interactions (Fig. 1). Its history is anchored in that of plant–plant interactions, although its realm has recently been extended to include other taxa1. Studies to date generally document its occurrence, and the ecological consequences of facilitation for individuals, species and ecosystems. The evolutionary causes and consequences of these interactions, though tackled using modelling approaches6 and phylogenetic analyses7, are still poorly assessed via contemporary evolution studies2. Despite the ubiquity of organisms that live within or upon others (endo or ecto-symbionts, hereafter ‘symbionts’ for brevity; Box 1), facilitation between symbionts has been largely overlooked. Indeed, individual hosts are often colonised by multiple symbionts, which can have positive, negative or neutral effects on one another (Fig. 1), independently of their effect on the host (e.g. either parasitic, commensal or mutualistic). Symbiont–symbiont competition has been shown both empirically and theoretically to have diverse effects on symbiont ecology and evolution8, but corresponding studies about facilitation in multiple infections (Box 1) are lacking. The host-symbiont literature contains many examples of symbiont–symbiont interactions compatible with facilitation (see Supplementary Table 1), though the interactions are rarely identified as such and are not unified into a common body of work. Moreover, a number of these studies investigate the evolutionary outcomes of facilitation9,10, which may be relevant for the interpretation of ecological patterns observed in both symbiotic and free-living systems.
Several reviews state that facilitative interactions have been relatively neglected in ecological theory, despite abundant empirical evidence for their occurrence in natural populations and indication of their importance for community functioning and stability1,2,5. Here, we argue that facilitation has been particularly overlooked in the symbiont literature4. The aim of this review is to highlight that integrating approaches used to study facilitation in free-living organisms with studies of symbionts will be highly informative and beneficial for both fields of research. On the one hand, placing symbiont–symbiont interactions in the context of facilitation should increase our understanding about infection outcomes. On the other hand, because it is easier to study evolution in symbionts than in most free-living organisms (given their shorter generation time), studies of symbiont–symbiont facilitation may guide predictions about how positive interactions between species could shape evolution in free-living communities.
First, we outline the different mechanisms of symbiont–symbiont facilitation. Next, we investigate the ecological and evolutionary conditions favouring the occurrence and maintenance of facilitation between symbiotic organisms. Finally, we discuss the ecological and evolutionary consequences of facilitation, and suggest future research avenues. Throughout, we highlight parallels with free-living organisms.
Mechanisms of facilitation
Symbionts can facilitate each other either directly (independently of the host) or indirectly (via host manipulation) and facilitation can occur both within- and between-hosts (Fig. 2). Overall, the mechanisms of facilitation between symbionts are similar to those found between free-living organisms (summarised in Fig. 3, along with some chosen examples; see also ref. 11, and Supplementary Table 1 for more examples of facilitation between symbionts).
Facilitation between symbionts can occur within or between hosts. Small blue circles: facilitating symbionts; small green circles: facilitated symbionts; big yellow circle: co-infected host; big grey circle: uninfected host; rings: entry/exit point of the host; blue dashed arrows: facilitation; black solid arrows: transition between life cycle stages of the facilitated symbiont. Traits facilitated are categorised as ‘development’ (i.e. symbiont growth or differentiation) in the within-host environment, ‘infection success’ (i.e. host entry or establishment), ‘exit from the host’ at the interface between the within- and between-host environment, and ‘transmission’ in the between-host environment. Note that the diagram does not consider the symmetry of the interaction (i.e. the effect of the facilitated symbiont on the facilitator is not represented)
Mechanisms underlying symbiont facilitation and parallels with free-living organisms. Blue circles: facilitating symbionts; green circles: facilitated symbionts; yellow shading: within-host environment; blue shading: between-host environment; grey or yellow circles: host; purple circles: resource; diamonds: host immune response; blue arrows: facilitation (solid: direct; dashed: indirect); black arrows: other types of effects (solid: enabled; dotted: cancelled). Traits facilitated are categorised as ‘development’ (i.e. symbiont’s growth or differentiation) in the within-host environment, ‘infection success’ (i.e. host entry or establishment) and ‘exit from the host’ at the interface between the within- and between-host environment, and ‘transmission’ in the between-host environment
Direct facilitation
Some symbionts directly facilitate the growth or reproduction of others, by producing substances aiding them to exploit the host (i.e. ‘supplied-product’ facilitation)12,13. Direct facilitation can occur when a symbiont facilitates another by affecting its gene expression (i.e. transactivation)14 or by providing essential gene products such as in the case of transcapsidation15 or helper component-transcomplementation15,16. Direct facilitation can also arise when exogenous genetic material from one symbiont becomes integrated in another (i.e. nested symbionts)17.
Indirect facilitation
Within-host indirect facilitation can be mediated by the modification of host resources used by symbionts18, or by improving host fitness in ways that benefit other symbionts. For instance, by increasing host longevity, a symbiont can reduce the survival cost of infection by another parasite19, which, in turn enhances the probability that the latter completes its development within the host.
Indirect facilitation also occurs via the host immune system. This can be brought about by immune-evasion strategies such as immunosuppression, which might be advantageous for other symbionts within the host14, or via immunological trade-offs, whereby a host is unable to simultaneously mount immune responses against different symbionts20,21,22. Furthermore, symbionts can facilitate host entry or exit of another4 via epidermal injuries23 or through the symptoms of infection24.
Finally, behavioural or reproductive manipulation of the host by a symbiont can facilitate the transmission (Box 1) of other symbionts (e.g. ‘hitch-hiking’)25. This might occur between horizontally transmitted symbionts with complex life cycles that share both intermediate and definitive hosts25, as well as between symbionts with different transmission routes26 (Box 1). Vertically transmitted reproductive manipulators that increase the proportion of infected female offspring in the population27 can also facilitate the transmission of other vertically transmitted symbionts (i.e. via synergy or hitchhiking)27,28.
Multiple mechanisms and multiple effects
Facilitation is often not easily attributed to a single mechanism. For example, HIV-1 triggers lymphocyte activation, and activated lymphocytes are the preferred resource of the human cytomegalovirus29. Hence, facilitation is immune-mediated from the facilitator perspective, but resource-mediated for the facilitated. Moreover, several facilitating mechanisms can operate simultaneously. For instance, the direct facilitation of polydnavirus transmission by parasitoid wasps described in Fig. 3 is accompanied by an indirect facilitation by the virus for wasps, via silencing of the host immune system17. Importantly, many (if not all) multiple infections entail some degree of competition between symbionts. This means that interactions between the same two organisms can be both facilitative and competitive, changing at different life-stages or in different environments11. Moreover, facilitation and competition can occur over the same resource/common good (Fig. 3), or facilitation might occur for one trait and competition for another30. For example, simultaneous infection of rabbits by two helminth species increases the density but decreases the fecundity of one symbiont, while having the opposite effect on the other31. Whether this can be considered as facilitation is debatable, and would ultimately require measuring the transmission of each parasite to new hosts, to ascertain whether the net direction of the interaction is negative or positive.
Similarly, it is not always clear whether competition or facilitation is the dominant interaction between a facilitator and a facilitated individual in plant species pairs32 (but see ref. 33). Consequently, the net effect of a facilitator on a facilitated results from unequal negative and positive effects, and some consider that the term ‘facilitation’ should be reserved for cases where positive effects dominate11. The interplay between competition and facilitation forms the basis of a classical prediction in the ecological literature, the stress-gradient hypothesis34: it states that positive interactions should be more frequent under more stressful environmental conditions, whereas negative interactions are expected to be more frequent under benign conditions34. A recent meta-analysis confirmed that competitive interactions declined with increasing stress between 727 pairs of plant species35. Whether this holds for pairs of symbiont species remains to be tested.
Another issue arises from potential correlations between symbiont life-history traits at the within/between-host level36. Indeed, a facilitator causing higher within-host symbiont growth might be assumed to also increase transmission (as these traits are often positively correlated and linked to fitness37). However, this does not always hold true. For instance, in Culex pipiens mosquitoes, Wolbachia increases Plasmodium relictum infection success and within-host growth, but not the number of transmissible stages38. Moreover, by increasing symbiont load, the presence of a facilitator can induce increased virulence (Box 1), which, in turn, might reduce transmission through a shortened infection duration37. Indeed, mathematical models parameterised from laboratory data show that facilitation measured as higher within-host growth does not always result in more severe epidemics18. Alternatively, the within-host effects of one symbiont (e.g. increased host fecundity leading to larger host populations) might facilitate another in the between-host environment (e.g. because there are more available hosts).
As highlighted by Alizon and Michalakis36, a fitness-based approach, which encompasses the entire symbiont life cycle, should be adopted when studying facilitation. This provides an estimate of total symbiont fitness rather than individual fitness components, and accounts for potential pleiotropic effects of facilitation on different traits (or hosts for multi-host symbionts26). Although it can be complicated to measure the number of secondary infections generated from singly vs multiply infected hosts, several system-specific alternatives can be used to estimate symbiont fitness36. This integrative approach should aid identifying the occurrence of facilitation (e.g. see Box 2), although it may be unrealistic to apply it to (long-lived) plants. For instance, Schöb et al.39 highlight that identifying costs of facilitation based on measures of reproductive output during one season fails to incorporate possible compensation via higher survival in long-lived facilitators.
Ecological and evolutionary drivers of symbiont–symbiont facilitation
Is facilitation ‘just’ ecological?
The literature on free-living organisms has identified a number of conditions favouring the occurrence of facilitation. We summarise these conditions for symbionts and draw parallels with free-living organisms in Table 1. The next question is whether these interactions persist for a sufficiently long time period, such that the facilitator may represent a selection pressure to which the facilitated can respond, and vice versa. The maintenance of, or selection for, facilitation between species thus requires that such organisms encounter one another frequently40,41. Factors increasing the likelihood that symbionts find themselves in the same host might be shared transmission routes27, or high prevalence in host populations42. In general, given the vast amount of evidence that has accumulated in recent decades showing that ecology and evolution operate at similar timescales (e.g. see ref. 43), it seems unreasonable to consider that facilitation will not be shaped by evolution. For instance, the stress-gradient hypothesis predicts the ecological conditions that favour facilitation34. If these conditions are constant during a given number of generations, this might select for responses in the facilitator to the facilitated, and vice versa. Similarly, it has been shown that facilitation among plants tends to occur among organisms that are more phylogenetically distant than expected by chance7,44. Once this interaction is established, and if sufficiently stable over time, it is likely to exert selection on the individuals involved.
Facilitation may not only be selected following its emergence, but its emergence may be driven by evolution due to kin selection or to resolve conflicts. For instance, a novel cooperative behaviour (fibril production enhancing group migration) evolved in experimental lineages of the soil bacterium Myxococcus xanthus45. Moreover, character displacement in response to competition46 may decrease functional overlap between two species, which in turn could increase the likelihood of facilitative interactions47. For example, in free-living bacteria, facilitation can emerge due to a niche shift in response to competition for a common resource when one species evolves to exploit the waste products of another48. Similarly, functional overlap between bacterial endosymbionts in the lachnid aphid may have selected for function partitioning and complementation, such as between Buchnera aphidicola and Serratia symbiotica (the former has lost certain genes whose functions are encoded by the latter)41.
Conditions favouring the evolution of facilitation
A main factor determining whether facilitation will evolve is the symmetry of the interaction2,39. In general, facilitation is predicted to be selected when the interaction is reciprocal (+/+; mutualism/cooperation), hence when the facilitator also experiences a net benefit from the interaction2. This is also predicted by inclusive-fitness models where cooperation between symbionts enables kin to exploit the environment and is thus selected in the presence of related individuals49,50,51. Similarly, persistent cooperation can occur between non-kin3 (see Box 3). For example, two different bacteriophages, in a +/+ relationship, evolved the co-packaging of their genomes for transmission to new hosts52. Unfortunately, this study was done with a single replicate selection line (i.e. there was no replication, as in experimental evolution replication is measured at the population/line level).
Facilitation might still evolve if it bears no cost to the facilitator (+/0; commensalism). In this case, the plant literature predicts that evolution should occur unilaterally in the facilitated species only2. The symbiont literature, in turn, predicts that, although facilitation is not adaptive per se, it might be indirectly selected if the trait leading to facilitation also benefits, or is genetically correlated with a trait that benefits, the facilitator53. For instance, facilitation via immunosuppression can be selected when it has pleiotropic beneficial effects on transmission53, but empirical evidence is lacking.
Finally, facilitation might evolve even when it negatively impacts the facilitator (+/−). In the plant literature, these interactions (e.g. nurse plant species negatively impacted by beneficiaries39) are predicted to exhibit lower evolutionary stability than mutualistic or commensal interactions, selecting for traits that eliminate associated costs; promoting tolerance or resistance to colonisation by the facilitated2, thereby switching the interaction towards +/0 or disappearing, respectively. Among symbionts, facilitation might be maintained if the benefit of host manipulation outweighs the cost imposed by the facilitated symbiont (see ‘Evolutionary dynamics of facilitation’ section below). Another possibility is that this type of interaction might produce an antagonistic, coevolutionary arms race between the facilitator and facilitated, as demonstrated between Staphylococcus aureus and Enterococcus faecalis over the exploitation of public goods10. These possibilities can be extended to plants and other free-living species, as equivalent studies are currently lacking in this area.
Moreover, interactions between symbionts and their host may also affect the probability that facilitation will evolve. Indeed, whether facilitation evolves more or less frequently among host parasites or among host mutualists remains an open question (see Box 4).
Evolutionary dynamics of facilitation
Facilitation between symbionts might not be permanent, such that the symmetry defined above (+/+, +/0 and +/−) is transitory. Consistent with this, public good models predict that cooperation should be counter-selected in the presence of non-kin49,50, or that facilitators should evolve to monopolise the resources they make available, thus shifting the interaction away from asymmetric facilitation54. However, in the latter case cheaters might also evolve to evade such monopolisation as shown in free-living systems55, again shifting the interaction towards asymmetric facilitation. Therefore, if players engage in arms races, facilitation might be an important force driving the evolutionary dynamics of a multiple infection even if it only occurs sporadically.
Such dynamical interactions, characterised by an arms race, might occur between herbivorous spider mite species, which make plants more suitable for themselves and competitors through down-regulation of plant defences56,57,58, but that also exclude competitors via highly localised down-regulation57 or the production of dense web59. Among plants, interactions switching between competition and facilitation due to environmental variation have been widely reported (reviewed in ref. 11). However, coevolutionary dynamics of facilitation between free-living organisms with asymmetric facilitation remains untested (Box 4). To our knowledge, the only example of experimental evolution of an asymmetric interaction shows facilitation loss after ten host generations9. Indeed, S. aureus coevolved with E. faecalis produced fewer siderophores (iron scavenging molecules) than the ancestral population and than S. aureus evolving alone9. This is probably because siderophores benefit E. faecalis, but the latter suppresses S. aureus growth9. However, corroboration of this conclusion would require experimental coevolution with mutant E. faecalis that do not benefit from siderophores (see Box 2).
Ecological and evolutionary consequences of facilitation
Persistence and diversification
Facilitation between free-living organisms can increase the range distribution of a facilitated species by allowing its recruitment into environments to which it is not adapted1. For instance, dryland vegetation simulation models show that facilitation extends regions in which facilitated species can survive due to resource concentration by facilitator species60. Similarly, banded mussel patterns are predicted to permit species persistence in otherwise food scarce conditions61. Niche extension due to facilitation may also increase species and phylogenetic diversity5,7. Moreover, selection for increased facilitation is predicted to reduce extinction risk in spatially subdivided populations with local, but not global, dispersal6. Whether such predictions also apply to facilitation between symbionts is a timely issue and has, to our knowledge, not been addressed. Indeed, both host range expansion and parasite diversity have important consequences for disease management.
Host health and virulence evolution
Knowledge stemming from studies of facilitation between free-living organisms could help predict the effects of facilitation between symbionts on host health. For instance, in harsh environments, facilitation can lead to the aggregation of individuals in space62, which, in turn, may increase ecosystem productivity compared to homogeneous distributions (e.g. mussel beds61 and dryland vegetation60). Moreover, empirical studies have shown that inter-specific facilitation can increase ecosystem functioning more generally34,63. This may also apply to facilitation between host mutualistic symbionts, which positively impact host health13,47.
These predictions from the literature on free-living organisms are, however, ignoring cases where facilitated species have negative effects on their environment. For instance, facilitation of invasive plant species (e.g. by other invasive plant species64, or herbivores65) may have deleterious consequences for ecosystem functioning reducing the biomass of resident species. Similarly, when facilitated symbionts are host parasites, laboratory observations show that facilitation often has negative impacts on host health (Supplementary Table 1). For example, a recent survey shows that multiple infections in humans can lead to higher parasite abundances and poorer host condition compared to single infections66. Likewise, mice infected with Bordatella bronchiseptica and Heligmosoides polygyris (see Supplementary Table 1) suffer higher mortality (accompanied by increased bacteria growth and helminth transmission) than those infected with a single species67. Another study shows that honey bee colony collapse might be attributed to immunosuppression caused by the mite Varroa destructor, promoting deformed wing virus growth, which is benign in single infections68.
Facilitation may also affect host health indirectly, via its effect upon virulence evolution. Facilitation is predicted to select for increased virulence by some theoretical models. Indeed, immunosuppression by one parasite might facilitate infection by a second, but subsequent competition for host resources can select for higher virulence30. Similarly, evolution of higher virulence is predicted in a superinfection scenario, where a first parasite facilitates infection and replacement by a second69 (this reduces infection duration, which selects for higher virulence). Finally, in inclusive fitness models, the production of public goods leads to elevated symbiont growth, which generally correlates positively with virulence49 (but see ref. 51).
Unfortunately, levels of virulence measured in empirical studies are not always the same as those predicted in theoretical models. Indeed, empirical studies usually measure virulence in individual hosts, within a single host generation (see examples in Table S1), whereas mathematical models analyse virulence evolution over several host generations at the population level8. Choisy and de Roode70 formalise this in a theoretical model predicting higher virulence in multiple infections that cause immunosuppression when symbionts have not evolved together, thus in the short-term. However, they also show that, in the long-term, evolution of this system selects for lower virulence, because of a longer infection duration in immunosuppressed hosts70. Lower virulence is also predicted to evolve when there is co-transmission of two symbionts (Box 1), one of them being a ‘helper’ required for transmission of the other71. This is because co-transmission, aligning the interests of co-infecting strains, decreases selection for increased within-host competition71.
The latter model illustrates the complex interplay between competition and facilitation that probably underlies most interactions between symbionts. As described earlier, an interaction is considered to be facilitation if its net effect is greater than that of competition. Virulence evolution will thus hinge upon the relative strength of these interactions, and the traits upon which they act. As for facilitation, competition between parasites in a multiple infection can select for higher or lower virulence8, or for the coexistence of strains with different levels of virulence72. A comprehensive understanding of virulence evolution due to facilitation thus also requires knowledge about the interplay between facilitation and competition, and underlying mechanisms. This could be investigated, for instance, in a system where the parasite life-stages engaging in each interaction are clearly defined (e.g. facilitation during the infection process followed by within-host competition for shared host resources30; see Boxes 2 and 4).
Furthermore, although host health is generally the read-out of virulence, it can be difficult to evaluate the relative contribution of each symbiont in multiple infections. This is easily measured when virulence and growth are correlated and when symbionts can be distinguished in a multiple infection8. However, virulence is not always related to symbiont growth, as assumed by most theoretical models, but might be due to immunopathology73,74. In such cases, symbionts in a multiple infection with facilitation might interact in such a way that virulence does not correspond to the sum of the virulence measured in each single infection. Similarly, the combined impact of host mutualists might be synergetic, additive or non-additive47.
Unfortunately, empirical tests of virulence evolution (i.e. across generations) in facilitating multiple infections are as yet extremely scarce (Box 2)8. Indeed, one study in which co-transmission evolved did not measure the virulence of the evolved viruses52. Others, consistent with inclusive fitness models49,50,51, found reduced virulence when bacteria producing public goods (e.g. Bt cry toxins produced by the Btk rifR strain of Bacillus thuringiensis75, or siderophores produced by S. aureus9) evolved in the presence of competitors that can also exploit them (e.g. B. thuringiensis Btt specR strain, or E. faecalis, respectively). These two systems could be exploited to disentangle the relative importance of competition versus facilitation on the evolution of virulence.
Effects on symbiont dynamics and disease management
Transmission and epidemiology
Facilitating symbionts can enhance the transmission of (other) pathogens. For example, for super-shedding events of HIV in humans24, and of helminth worms in mice67, multiply infected individual hosts shed disproportionate numbers of transmissible stages. Moreover, transient effects of facilitation might enable a symbiont to exceed an ‘outbreak threshold’76, and cause epidemics even after facilitation has abated. However, true understanding of the long-term consequences of facilitation for symbiont epidemiology remains elusive.
Effects of facilitation on epidemiology have been demonstrated via long-term tracking of multiple infections in field populations. Indeed, one 5-year study, showed that the probability of infection was often higher for voles already harbouring another symbiont compared to uninfected individuals40. Alternatively, field observations can be corroborated with common garden experiments measuring symbiont transmission. For example, the transmission of the fungus Podosphaera plantaginis was higher from multiply versus singly infected Plantago lanceolata plants, which might explain the more severe epidemics observed in populations harbouring multiple strains77. Moreover, epidemiological models parameterised with empirical data show that predicted patterns coincide with field observations: positive interactions between symbionts can enhance their spread42,78. This approach has highlighted that reciprocal positive interactions between HIV and malaria in multiply infected hosts promote the spread of both parasites42, and that influenza can cause a higher incidence of pneumonia in human populations78. Unfortunately, theoretical models generating hypotheses on the long-term epidemiological consequences of facilitation are still scarce (but see ref. 79).
Disease management
As facilitation between symbionts can promote host range expansion, parasite diversity and epidemics, exacerbate the negative effects of infection on host health and impact virulence evolution, identifying whether certain symbionts are broad-acting facilitators might, in the future, aid the development of effective control strategies. Indeed, biocontrol microorganisms80 might be improved if their facilitators are also introduced into risk areas. Moreover, promoting facilitation between mutualist symbionts, such as those of the gut microbiota, that increase general host health13 or prevent infection with parasitic symbionts80, could also be considered. However, such strategies should be approached with caution, as they may also entail undesirable effects. For example, facilitation of gut microbiota can lead to increased pathogenicity in normally commensal bacteria81. Therefore, understanding facilitation might also unveil unforeseen consequences of pest management.
One could also think about alternative strategies, aside from conventional methods of pest or parasite control, to prevent the occurrence of facilitation or facilitators, thereby controlling facilitated parasites. This might be achieved by changing the biotic or abiotic environment such that facilitation is prevented. For instance, the release of cheaters into parasite populations to counter-select strains engaged in social, facilitating behaviours linked with virulence has been suggested82,83. However, these strategies might be ineffective due to spatial segregation of facilitator and facilitated strains, or to phenotypic plasticity82,83. For instance, if phenotypic plasticity is at play, behaviours associated with facilitation and virulence (e.g. public good production) might be arrested, rather than counter-selected, in the presence of an introduced cheat83 (Box 4).
Summary and outlook
Facilitation improves understanding of host–pathogen interactions
Recent reviews highlight the importance of extending the one host—one symbiont paradigm by studying symbiont interactions in a community ecology context (e.g. see refs. 84,85,84,86). However, facilitative interactions among symbionts have only recently gained attention (e.g. see refs. 27,81), which is at odds with the broad knowledge from the plant literature. Indeed, it is clear that many open questions remain (Box 4). For instance, what conditions favour the evolution and maintenance of facilitation among symbionts (host mutualists or parasites, and/or the frequency of multiply infected hosts). And what are the longer-term consequences of facilitation? Does coevolution between facilitating and facilitated symbionts lead to reduced facilitation, or to a coevolutionary arms race? Finally, how does symbiont–symbiont facilitation affect epidemiology (transmission), and virulence evolution?
Hence, facilitation may shed light on the study of host–parasite interactions. In this sense, the extensive knowledge acquired from more than thirty years on ecological facilitation among plants may constitute a solid theoretical base to investigate the complexity of symbiont interactions and their consequences for hosts.
Symbiont facilitation informs community ecology and evolution
Understanding facilitative interactions between symbionts might also inform on general principles in community ecology. For example, facilitation is predicted to improve ecosystem functioning63, to increase productivity at the community scale, and to expand the realised niche of maladapted species1,5. Such predictions have been corroborated by empirical studies44, but they would gain from being tested in manipulative experiments. As hosts are a contained environment that can harbour multiple symbiont species and can be tightly controlled and manipulated, they provide an excellent arena to establish causality, and critically test the abovementioned ideas with replication at the community level.
Symbionts can also shed new light on understanding how evolution shapes facilitation. For instance, research on facilitation among free-living organisms has ignored the possibility of an arms race between facilitators and facilitated2,39. Placing symbionts at the core of the facilitation literature allows not only considering, but also testing this possibility. Furthermore, given the similar timescales at which ecology and evolution operate, facilitation may be shaped by eco-evolutionary feedbacks. These may not be uncovered in other systems due to long generation times, such as long-lived plant species39. The typical short generation time of symbionts and their consequent amenability to experimental (co)evolution (Box 2) make them excellent candidates to address the (eco-)evolutionary consequences of facilitation at several scales.
Therefore, in the study of facilitation, as in many other areas, it is crucial to build bridges between contrasting fields of research, to generate fruitful positive feedbacks at different levels and to open new research avenues.
Proposed experimental design to test the evolution of facilitation. a Experimental design required to measure evolved responses to facilitation. Small green circles: facilitated symbionts (FD); small blue circles: facilitator symbionts (FR); big grey circles: hosts. Bold coloured arrows: (co)evolving players; thin black arrows: naive players (i.e. introduced from a naive base population at each generation). Treatments: (i) evolution of the facilitator in single infections: FR(ES); (ii) evolution of the facilitator in multiple infections: FR(EM); (iii) coevolution of the facilitated and facilitator in multiple infection: FR(CM) and FD(CM), respectively; (iv) evolution of the facilitated in multiple infections: FD(EM); (v) evolution of the facilitated in single infections: FD(ES). b Example of a possible evolved response of local adaptation in which facilitated symbionts have higher fitness when assayed in the infection environment in which they evolved. (1) Facilitation; (2) adaptation to the presence of a facilitator; (3) adaptation to the presence of a coevolved facilitator; (4) cost of adaptation to the facilitator; (5) local adaptation to either a coevolved or evolved facilitator
References
Bruno, J. F., Stachowicz, J. J. & Bertness, M. D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125 (2003). A key review article stating that the role of facilitation driving ecological interactions had been greatly underappreciated in the ecological literature.
Bronstein, J. L. The evolution of facilitation and mutualism. J. Ecol. 97, 1160–1170 (2009). Review article postulating the importance of evolution for facilitation among plants.
Barker, J. L. et al. Synthesizing perspectives on the evolution of cooperation within and between species. Evolution 71, 814–825 (2017). Review article highlighting the similarities between intra and inter-specific cooperation along five different axes.
Hellard, E., Fouchet, D., Vavre, F. & Pontier, D. Parasite–parasite interactions in the wild: how to detect them? Trends Parasitol. 31, 640–652 (2015).
Stachowicz, J. J. Mutualism, facilitation, and the structure of ecological communities. Bioscience 51, 235–246 (2001).
Kéfi, S., van Baalen, M., Rietkerk, M. & Loreau, M. Evolution of local facilitation in arid ecosystems. Am. Nat. 172, E1–E17 (2008).
Valiente-Banuet, A. & Verdú, M. Facilitation can increase the phylogenetic diversity of plant communities. Ecol. Lett. 10, 1029–1036 (2007).
Alizon, S., de Roode, J. C. & Michalakis, Y. Multiple infections and the evolution of virulence. Ecol. Lett. 16, 556–567 (2013). Review article synthesising the theoretical and experimental consequences of competition between symbionts in multiple infections.
Ford, S. A., Kao, D., Williams, D. & King, K. C. Evolution of reduced pathogen virulence. Nat. Commun. 7, 1–9 (2016). Experimental study showing that coevolution between different bacterial species can select for reduced virulence.
Ford, S. A., Williams, D., Paterson, S. & King, K. C. Co-evolutionary dynamics between a defensive microbe and a pathogen driven by fluctuating selection. Mol. Ecol. 26, 1778–1789 (2017).
Callaway, R. Positive Interactions and Interdependence in Plant Communities (Springer, 2007). A book reviewing the evidence for the role of inter-specific facilitation in plant communities, the mechanisms involved and the functional consequences.
Woyke, T. et al. Symbiosis insights through metagenomic analysis of a microbial consortium. Nature 443, 950–955 (2006).
Rakoff-Nahoum, S. et al. The evolution of cooperation within the gut microbiota. Nature 533, 255–259 (2016).
Van de Perre, P. et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect. Dis. 8, 490–497 (2008).
Syller, J. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol. Plant Pathol. 13, 204–216 (2012).
Froissart, R., Michalakis, Y. & Blanc, S. Helper component-transcomplementation in the vector transmission of plant viruses. Phytopathology 92, 576–579 (2002).
Herniou, E. A. et al. When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses. Philos. Trans. R. Soc. B 368, 20130051 (2013).
Ramiro, R. S., Pollitt, L. C., Mideo, N. & Reece, S. E. Facilitation through altered resource availability in a mixed-species rodent malaria infection. Ecol. Lett. 19, 1041–1050 (2016). Experimental and theoretical study showing that facilitation between different species of malaria increases virulence in multiply infected hosts, and that facilitation can increase the prevalence of the facilitated species.
Zélé, F., Nicot, a, Duron, O. & Rivero, A. Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. J. Evol. Biol. 25, 1243–1252 (2012).
Abe, H. et al. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 53, 204–212 (2012).
Belliure, B., Janssen, A., Maris, P. C., Peters, D. & Sabelis, M. W. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70–79 (2005).
Graham, A. L. Ecological rules governing helminth-microparasite coinfection. Proc. Natl Acad. Sci. USA 105, 566–570 (2008). Metaanalysis showing how ressource- and immune-mediated mechanisms mediate facilitative, neutral or competitive interactions across a broad spectrum of parasite taxa in laboratory mice.
Swe, P. M., Zakrzewski, M., Kelly, A., Krause, L. & Fischer, K. Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PLoS Negl. Trop. Dis. 8, 11–14 (2014).
Cohen, M. S. et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349, 1868–1873 (1997).
Cézilly, F., Perrot-Minnot, M. & Rigaud, T. Cooperation and conflict in host manipulation: interactions among macro-parasites and micro-organisms. Front. Microbiol. 5, 1–10 (2014).
Rode, N. et al. Why join groups? Lessons from parasite-manipulated Artemia. Ecol. Lett. 16, 493–501 (2013).
Vautrin, E. & Vavre, F. Interactions between vertically transmitted symbionts: cooperation or conflict? Trends Microbiol. 17, 95–99 (2009).
Zhao, D., Chen, D., Ge, C., Gotoh, T. & Hong, X. Multiple infections with Cardinium and two strains of Wolbachia in the spider mite Tetranychus phaselus Ehara: revealing new forces driving the spread of Wolbachia. PLoS. One. 8, e54964 (2013).
Biancotto, A. et al. Upregulation of human cytomegalovirus by HIV type 1 in human lymphoid tissue ex vivo. AIDS Res. Hum. Retrovir. 24, 453–462 (2008).
Kamiya, T., Mideo, N. & Alizon, S. Co-evolution of virulence and immunosuppression through multiple infections. J. Evol. Biol. 31, 995–1005 (2018). peer reviewed by Peer Community In Evolutionary Biology, 149211 Theoretical study showing that simultaneous facilitation (aiding infection) and competition (for shared host resources) between parasites can select for elevated virulence.
Cattadori, I. et al. Infections do not predict shedding in co-infections with two helminths from a natural system. Ecology 95, 1684–1692 (2014).
Callaway, R., Nadkarni, N. & Mahall, B. Facilitation and interference of Quercus douglasii on understory productivity in Central California. Ecology 72, 1484–1499 (1991).
Maestre, F. T., Bautista, S. & Cortina, J. Positive, negative, and net effect in grass-shrubs interactions in Mediterranean semiarid grasslands. Ecology 84, 3186–3187 (2003).
Michalet, R. et al. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 9, 767–773 (2006).
He, Q., Bertness, M. D. & Altieri, A. H. Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 16, 695–706 (2013).
Alizon, S. & Michalakis, Y. Adaptive virulence evolution: the good old fitness-based approach. Trends Ecol. Evol. 30, 248–254 (2015).
Anderson, R. M. & May, R. M. Coevolution of hosts and parasites. Parasitology 85, 411–426 (1982).
Zélé, F. et al. Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc. R. Soc. B 281, 20132837 (2014).
Schöb, C. et al. A global analysis of bidirectional interactions in alpine plant communities shows facilitators experiencing strong reciprocal fitness costs. New Phytol. 202, 95–105 (2014).
Telfer, S. et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246 (2010).
Douglas, A. E. How multi-partner endosymbioses function. Nat. Rev. Microbiol. 14, 731–743 (2016).
Abu-Raddad, L., Patnaik, P. & Kublin, J. Dual infection with HIV and malaria fuels the spread of both diseases in Sub-Saharan Africa. Science 314, 1603–1606 (2006).
Fussmann, G. F., Loreau, M. & Abrams, P. A. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 21, 465–477 (2007).
Valiente-Banuet, A., Vital Rumebe, A., Verdu, M. & Callaway, R. M. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proc. Natl. Acad. Sci. USA. 103,16821–16817 (2006).
Velicer, G. J. & Yu, Y. T. N. Evolution of novel cooperative swarming in the bacterium Myxococcus xanthus. Nature 425, 75–78 (2003). Experimental study showing the evolution of facilitation (cooperative behaviour) between free-living bacteria.
MacArthur, R. H. & Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385 (1967).
Afkhami, M. E., Rudgers, J. A. & Stachowicz, J. J. Multiple mutualist effects: conflict and synergy in multispecies mutualisms. Ecology 95, 833–844 (2014).
Lawrence, D. et al. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 10, e1001330 (2012).
West, S. A. & Buckling, A. Cooperation, virulence and siderophore production in bacterial parasites. Proc. R. Soc. B 270, 37–44 (2003).
Brown, S. P. & Johnstone, R. A. Cooperation in the dark: signalling and collective action in quorum-sensing bacteria. Proc. R. Soc. B 268, 961–965 (2001).
Alizon, S. & Lion, S. Within-host parasite cooperation and the evolution of virulence. Proc. R. Soc. B 278, 3738–3747 (2011).
Sachs, J. L. & Bull, J. J. Experimental evolution of conflict mediation between genomes. Proc. Natl Acad. Sci. USA 102, 390–395 (2005).
Bonhoeffer, S. & Nowak, M. A. Intra-host versus inter-host selection: Viral strategies of immune function impairment. Proc. Natl Acad. Sci. USA 91, 8062–8066 (1994).
Krakauer, D. C., Page, K. M. & Erwin, D. H. Diversity, dilemmas, and monopolies of niche construction. Am. Nat. 173, 26–40 (2009).
Kummerli, R. et al. Co-evolutionary dynamics between public good producers and cheats in the bacterium Pseudomonas aeruginosa. J. Evol. Biol. 28, 2264–2274 (2015).
Godinho, D. P., Janssen, A., Dias, T., Cruz, C. & Magalhães, S. Down-regulation of plant defence in a resident spider mite species and its effect upon con- and heterospecifics. Oecologia 180, 161–167 (2016).
Glas, J. J. et al. Defense suppression benefits herbivores that have a monopoly on their feeding site but can backfire within natural communities. BMC Biol. 12, 12–98 (2014).
Sarmento, R. A. et al. (2011) A herbivore that manipulates plant defence. Ecol. Lett. 14, 229–236 (2011).
Sarmento, R. et al. A herbivorous mite down-regulates plant defence and produces web to exclude competitors. PLoS. ONE. 6, e23757 (2011).
Kéfi, S., Rietkerk, M., van Baalen, M. & Loreau, M. Local facilitation, bistability and transitions in arid ecosystems. Theor. Popul. Biol. 71, 367–379 (2007).
van de Koppel, J., Rietkerk, M., Dankers, N. & Herman, P. M. J. Scale-dependent feedback and regular spatial patterns in young mussel beds. Am. Nat. 165, E66–E77 (2005).
van de Koppel, J. et al. Experimental evidence for spatial self-organization and its emergent effects in mussel bed ecosystems. Science 322, 739–742 (2008).
Cardinale, B. J., Palmer, M. A. & Collins, S. L. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415, 426–429 (2002).
Flory, S. L. & Bauer, J. T. Experimental evidence for indirect facilitation among invasive plants. J. Ecol. 102, 12–18 (2014).
Blossey, B. & Gorchov, D. L. Ungulates and invasive species: quantifying impacts and understanding interactions. AoB Plants 9, 1–6 (2017).
Griffiths, E. C., Pedersen, A. B., Fenton, A. & Petchey, O. L. The nature and consequences of coinfection in humans. J. Infect. 63, 200–206 (2011).
Lass, S. et al. Generating super-shedders: co-infection increases bacterial load and egg production of a gastrointestinal helminth. J. R. Soc. Interface 10, 20120588 (2012).
Nazzi, F. et al. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Biol. 8, e1002735 (2012).
Kada, S. & Lion, S. Superinfection and the coevolution of parasite virulence and host recovery. J. Evol. Biol. 28, 2285–2299 (2015).
Choisy, M. & de Roode, J. C. Mixed infections and the evolution of virulence: effects of resource competition, parasite plasticity, and impaired host immunity. Am. Nat. 175, E105–E118 (2010). Theoretical study showing that facilitation between parasites via host immunosuppression can select for reduced virulence due to a longer infection duration.
Alizon, S. Parasite co-transmission and the evolutionary epidemiology of virulence. Evolution 67, 921–933 (2013).
Alizon, S. & van Baalen, M. Multiple infections, immune dynamics, and the evolution of virulence. Am. Nat. 172, E150–E168 (2008).
Graham, A. L., Allen, J. E. & Read, A. F. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Syst. 36, 373–397 (2005).
Weigert, M. et al. Manipulating virulence factor availability can have complex consequences for infections. Evol. Appl. 10, 91–101 (2017).
Garbutt, J., Bonsall, M. B., Wright, D. J. & Raymond, B. Antagonistic competition moderates virulence in Bacillus thuringiensis. Ecol. Lett. 14, 765–772 (2011).
Hartfield, M. & Alizon, S. Introducing the outbreak threshold in epidemiology. PLoS Biol. 9, 9–12 (2013).
Susi, H., Barrès, B., Vale, P. F. & Laine, A.-L. Co-infection alters population dynamics of infectious disease. Nat. Commun. 6, 5975 (2015).
Shrestha, S., Foxman, B., Berus, J. & Panhuis, W. G. Van The role of influenza in the epidemiology of pneumonia. Sci. Rep. 5, 15314 (2015).
Sofonea, M. T., Alizon, S. & Michalakis, Y. Exposing the diversity of multiple infection patterns. J. Theor. Biol. 419, 278–289 (2017).
McNally, L. & Brown, S. P. Building the microbiome in health and disease: niche construction and social conflict in bacteria. Philos. Trans. R. Soc. B 370, 20140298 (2015).
Almand, E. A., Moore, M. D. & Jaykus, L. A. Virus-bacteria interactions: an emerging topic in human infection. Viruses 9, 1–10 (2017).
Brown, S., West, S. A., Stephen, P. & Griffin, A. S. Social evolution in micro-organisms and a Trojan horse approach to medical intervention strategies. Proc. R. Soc. B 364, 3157–3168 (2009).
Leggett, H. C., Brown, S. P. & Reece, S. E. War and peace: social interactions in infections. Philos. Trans. R. Soc. B 369, 20130365 (2014).
Johnson, P. T. J., de Roode, J. C. & Fenton, A. Why infectious disease research needs community ecology. Science 349, 1259504-1–1259504-9 (2015).
Pedersen, A. B. & Fenton, A. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 22, 133–139 (2007).
Seabloom, E. W. et al. The community ecology of pathogens: coinfection, coexistence and community composition. Ecol. Lett. 18, 401–415 (2015).
Heil, M. & Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 25, 137–144 (2010).
Márquez, R., Corredor, G., Galvis, C., Góes, D. & Amézquita, A. Range extension of the critically endangered true poison-dart frog, Phyllobates terribilis (Anura: Dendrobatidae), in western Colombia Acta Herpetol. 7, 341–345 (2012).
Greenwood, P. G. Acquisition and use of nematocysts by cnidarian predators. Toxicon 54, 1065–1070 (2009).
Bhattacharya, D., Pelletreau, K. N., Price, D. C., Sarver, K. E. & Rumpho, M. E. Genome analysis of Elysia chlorotica egg DNA provides no evidence for horizontal gene transfer into the germ line of this kleptoplastic mollusc.Mol. Biol. 30, 1843–1852 (2013).
Soliveres, S., Smit, C. & Maestre, F. T. Moving forward on facilitation research: response to changing environments and effects on the diversity, functioning and evolution of plant communities. Biol. Rev. Camb. Philos. Soc. 90, 297–313 (2015).
John, U. et al. Intraspecific facilitation by allelochemical mediated grazing protection within a toxigenic dinoflagellate population. Proc. R. Soc. B 282, 20141268 (2015).
Long, W. C., Gamelin, E. F., Johnson, E. G. & Hines, A. H. Density-dependent indirect effects: apparent mutualism and apparent competition coexist in a two-prey system. Mar. Ecol. Prog. Ser. 456, 139–148 (2012).
Odadi, W. O., Karachi, M. K., Abdulrazak, S. A. & Young, T. P. African wild ungulates compete with or facilitate cattle depending on season. Science 333, 2–4 (2011).
Loder, M., Boersma, M., Kraberg, A., Aberle, N. & Wiltshire, K. Microbial predators promote their competitors: commensalism within an intra-guild predation system in microzooplankton. Ecosphere 5, 128 (2014).
Gordon, I. Facilitation of red deer grazing by cattle and its impact on red deer performance. J. Appl. Ecol. 25, 1–9 (1998).
Orsucci, M., Navajas, M. & Fellous, S. Genotype-specific interactions between parasitic arthropods. Heredity 118, 260–265 (2016).
Hughes, A. R., Moore, A. F. P. & Piehler, M. F. Independent and interactive effects of two facilitators on their habitat-providing host plant. Spartina Alter. Oikos 123, 488–499 (2014).
Klemme, I., Louhi, K. R. & Karvonen, A. Host infection history modifies co-infection success of multiple parasite genotypes. J. Anim. Ecol. 85, 591–597 (2016).
Fukami, T., Bezemer, T., Mortimer, S. & van der Putten, W. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 8, 1283–1290 (2005).
Acknowledgements
We would like to dedicate this paper to the memory of Isabelle Olivieri, for being such a great example of a person and a scientist and for having influenced us in so many ways that enumerating them would not fit within the word limit of this Article. We would like to thank Adrián Escudero, Alain Danet, Alex Hall, Franck Prugnolle, Rolf Kümmerli, Virginie Rougeron and Yannis Michalakis for very helpful comments, which have greatly improved the manuscript. This work was funded by an ERC consolidating grant (COMPCON GA 725419) to S.M. and by a PHC-PESSOA grant (38014YC) to S.M. and A.B.D. F.Z. was funded through FCT Post-Doc fellowship (SFRH/BPD/125020/2016). This is ISEM contribution number 2018-165.
Author information
Authors and Affiliations
Contributions
All authors participated in writing the entire manuscript. S.K. mostly contributed sections about facilitation in free-living organisms, and F.Z., S.M. and A.B.D. mostly sections about facilitation between symbionts. F.Z. designed the figures. A.B.D. conceived of the original idea for this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zélé, F., Magalhães, S., Kéfi, S. et al. Ecology and evolution of facilitation among symbionts. Nat Commun 9, 4869 (2018). https://doi.org/10.1038/s41467-018-06779-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-06779-w
This article is cited by
-
Metabolic cooperation between conspecific genotypic groups contributes to bacterial fitness
ISME Communications (2023)
-
Reciprocal positive effects on parasitemia between coinfecting haemosporidian parasites in house sparrows
BMC Ecology and Evolution (2022)
-
Influence of bacteria on the maintenance of a yeast during Drosophila melanogaster metamorphosis
Animal Microbiome (2021)
-
Reciprocal interactions between tumour cell populations enhance growth and reduce radiation sensitivity in prostate cancer
Communications Biology (2021)
-
Habitat loss causes long extinction transients in small trophic chains
Theoretical Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.