Abstract
Recently, the development of high-performance non-platinum electrocatalysts for fuel cell applications has been gaining attention. Palladium-based nanoalloys are considered as promising candidates to substitute platinum catalysts for cathodic and anodic reactions in fuel cells. Here, we develop a facile route to synthesize dendritic palladium–copper–cobalt trimetallic nanoalloys as robust multifunctional electrocatalysts for oxygen reduction and formic acid oxidation. To the best of our knowledge, the mass activities of the dendritic Pd59Cu30Co11 nanoalloy toward oxygen reduction and formic acid oxidation are higher than those previously reported for non-platinum metal nanocatalysts. The Pd59Cu30Co11 nanoalloys also exhibit superior durability for oxygen reduction and formic acid oxidation as well as good antimethanol/ethanol interference ability compared to a commercial platinum/carbon catalyst. The high performance of the dendritic Pd59Cu30Co11 nanoalloys is attributed to a combination of effects, including defects, a synergistic effect, change of d-band center of palladium, and surface strain.
Similar content being viewed by others
Introduction
Fuel cells (FCs) are efficient, clean, and sustainable energy generation units that produce electricity from fuels such as hydrogen, formic acid, methanol, and ethanol1,2,3. Platinum (Pt) is widely applied as a catalyst for anode and cathode reactions in FCs4,5,6,7,8, but many factors, including high cost that accounts for over 55% of the total cost9, scarcity, poor durability, and sluggish reaction kinetics of the oxygen reduction reaction (ORR), tremendously impede the commercial application of FCs. Therefore, developing highly effective non-Pt alloy catalysts for FCs has aroused great interest and concern of researchers from all over the world since such alloys can enhance the catalytic performance through optimizing the binding energy between reactants, intermediates and products with the alloy surface at the nanoscale10,11,12,13. For instance, Linic’s group, Yoo’s group, and Yang’s group, respectively reported Ag–Co alloy, Rh–Sn alloy, Pd–Rh alloy, and Au–Rh alloy catalysts for electrochemical ORR in basic media14,15,16,17,18. However, most of the mass activities reported on non-Pt metal catalysts are below 0.20 A mg−1 for ORR, which is still far from the target (0.44 A mg−1Pt at 0.9 V reversible hydrogen electrode (RHE)) of the U.S. Department of Energy19. Besides activities, the alcohol (methanol or ethanol) tolerance of metal catalysts is an important concern for direct alcohol (methanol or ethanol) FCs since these fuel molecules generally penetrate the polymer electrolyte membrane and diffuse from the anode to the cathode, which will greatly lower the performance and efficiency of metal catalysts. However, no alcohol tolerance experiments were mentioned on aforementioned non-Pt metal catalysts.
Among non-Pt multimetallic electrocatalysts, Pd-based electrocataysts have received ample attention for organic molecular oxidation and ORR20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39. Previous reports have shown that Pd-based nanoalloys can exhibit superior catalytic performance relative to Pt catalysts for FC applications40,41,42,43. As we know, the size, composition and complex shape (e.g., a dendritic shape) can be used to tune the electrocatalytic performance of Pd-based catalysts. Furthermore, many parameters, such as Pd–Pd interatomic distance, the number of Pd nearest neighbors, the d-band center of Pd and the Pd content on the nanoalloy surface, will change when Pd alloys with 3d transition metals10,12,13. Therefore, rational regulation of these parameters can boost the catalytic activity and/or durability of Pd-based nanocatalysts for ORR and/or small organic molecular oxidation. For instance, Han’s group40 and Huang’s group43 have, respectively, displayed PdCuCo anisotropic structure and ordered spherical PdCuCo with mass activities at 0.9 V versus RHE of 0.18 and 0.13 A mg−1Pd for ORR, but no alcohol tolerance experiments were mentioned. Xing’s group has reported that the mass activity (MA) of palladium–cobalt phosphorus/carbon (Pd–CoP/C) is 2.757 A mg−1Pd toward formic acid oxidation (FAO)21. Zhang’s group has reported that 4H/face-centered cubic (fcc) Au@Pd core–shell nanorods exhibit high activity toward ethanol oxidation (EO)24. However, to the best of our knowledge, there is no report on the synthesis of trimetallic PdCuCo dendritic nanoalloys as robust multifunctional electrocatalysts for both ORR and FAO.
Herein, we report a facile synthesis of trimetallic PdCuCo dendritic nanoalloys with abundant defects. The electrocatalytic performance of these as-synthesized dendritic PdCuCo nanoalloys toward ORR and FAO has been investigated. Compared with commercial Pt/C or Pd black, the dendritic Pd59Cu30Co11 nanoalloys demonstrate much higher MA and durability. Besides, the dendritic Pd59Cu30Co11 nanoalloys also exhibit high alcohol (methanol or ethanol) tolerance compared with commercial Pt/C. Thus, this approach provides an effective route for fabricating dentritic PdCuCo nanoalloys as robust multifunctional electrocatalysts for ORR and FAO by creating abundant defects.
Results
Structural characterization
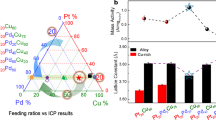
Figure 1a, b and Supplementary Fig. 1a showed the representative transmission electron microscopy (TEM) images of the as-synthesized Pd59Cu30Co11 nanocrystals (the composition was determined by the inductively coupled plasma optical emission spectrometry (ICP–OES), Supplementary Table 1). As can be seen, the obtained products were of uniform size of 66.25 ± 4.5 nm (Supplementary Fig. 1b) and displayed dendritic shapes. These dendritic particles were assembled with dozens of small grains, and the size of most small grains was sub-5.0 nm, around 4.28 nm (Supplementary Fig. 1c). Well-resolved lattice fringes (Fig. 1c) are observed in the sub-5.0 nm grains and the lattice distance of 0.217 nm is very closed to the (111) interplanar distance of face-centered cubic (fcc) Pd. The energy dispersive X-ray spectroscopy data of one single dendritic particle showed that the as-synthesized dendritic particle consists of Pd, Cu, and Co (Supplementary Fig. 1d). The X-ray diffraction (XRD) patterns (Supplementary Fig. 1e) of the as-synthesized Pd59Cu30Co11 nanocrystals showed four peaks located at 40.45, 47.05, 68.70, and 81.85° 2θ that can be indexed to (111), (200), (220), and (311) planes of the fcc Pd (JCPDS-46-1403)44, respectively. The diffraction pattern is not characteristic of fcc Cu (JCPDS-04-0836)23 and fcc Co (JCPDS-15-0806)45 phases. The peak positions of Pd50Cu50, Pd88Co12, and Pd59Cu30Co11 are shifted to higher angles relative to those of the pure Pd crystal (JCPDS-46-1403), indicating that the smaller Cu and Co atoms are incorporated into the Pd lattice. According to the XRD patterns and the Debye–Scherrer equation46 (Table 1), Cu and/or Co entering the Pd lattice can induce lattice contraction and strain variation, and the strain variations of trimetallic PdCuCo nanoalloys (Pd59Cu30Co11:3.50%; Pd56Cu38Co6:2.56%; Pd62Cu16Co22:2.11%) are higher than that of bimetallic Pd50Cu50 (1.62%) and Pd88Co12 (0.72%), which indicates that simultaneously introducing Cu and Co atoms should result in greater strain variation than introducing only Cu or Co in current system and would enhance catalytic performance of nanocrystals12,13,39,40,43. The results of XRD indicated the formation of Pd, Cu, and Co nanoalloys. Moreover, the nanoalloy structure of the as-synthesized Pd59Cu30Co11 nanodendrites was further confirmed by aberration-corrected high-resolution elemental mapping analysis (Fig. 1d–h). The elemental mapping of Pd59Cu30Co11, showed that the Pd, Cu, and Co distributed throughout the whole particle (Fig. 1e–h). Simultaneously, the Pd, Cu, and Co atoms were verified to coexist in the topmost atomic layer within near-surface of the as-synthesized Pd59Cu30Co11 nanodendrite and Pd atoms neighbored Cu and Co atoms. The aberration-corrected high-resolution TEM was further used to analyze the surface structure of the as-synthesized Pd59Cu30Co11 nanodendrite. As shown in Fig. 2, abundant defects including low-coordination number (edges, corners, and steps) atoms, grain boundaries, lattice disorder, gap atoms, vacancies, and nanotwins were clearly observed in the surface. These defects have been confirmed to act as highly active sites and can boost the catalytic performance of catalysts in catalytic reaction4,19,47,48,49,50,51.
Characterization of the as-synthesized Pd59Cu30Co11 nanoalloys. The typical transmission electron microscopy (TEM) (a, b), the high-resolution TEM (HRTEM) (c) images, the aberration-corrected high-resolution high-angle annular-dark field scanning transmission electron microscopy (HAADF-STEM) (d) images and the corresponding energy dispersive X-ray spectroscopy (EDS) elemental mapping (e–h) images of the as-synthesized Pd59Cu30Co11 nanoalloys. (Scale bar: a is 50 nm, b is 20 nm, c, d are 5 nm.)
Surface defect characterization of the as-synthesized Pd59Cu30Co11 nanoalloys. Defect analysis images of the as-synthesized dendritic Pd59Cu30Co11 nanoalloys through aberration-corrected transmission electron microscopy (TEM). a, b Edge, corner and lattice disorder (in yellow dashed circle); c step; d gap atom in yellow dashed circle and vacancy in red dashed circle; e, f Nanotwins. (Scale bar: a, b are 5 nm, c–f are 2 nm.)
X-ray photoelectron spectroscopy (XPS) is generally accepted to analyze the surface composition and state of nanocrystal surface within 2.0 nm. The XPS results (Fig. 3) of the as-synthesized Pd59Cu30Co11 showed that the Pd, Cu, and Co atoms coexisted in the near-surface of Pd59Cu30Co11nanoalloys. The binding energy of the Pd 3d peaks (Fig. 3a) shifted to 335.5 and 340.7 eV compared to the standard Pd 3d peaks (335.2 and 340.4 eV), and the binding energy of metallic Pd in the nanosurface of Pd59Cu30Co11 nanoalloys positively shifted about 0.3 eV, which meant the downshift of the d-band center of Pd and this result was in accordance with aforementioned reports13,46,52,53. To further probe the local structure of the Pd59Cu30Co11 nanoalloys at atomic scale, the X-ray absorption fine structure measurements were performed at the Pd K-edge. From the X-ray absorption near edge structure of the Pd K-edge (Fig. 3d), we can clearly see that the Pd K-edge curve of the Pd59Cu30Co11 nanoalloys was almost the same as that of metal state of Pd-foil and was quite different from that of the PdO, indicating that the as-synthesized Pd59Cu30Co11 did not contain the PdO. The binding energy of Cu 2p peaks (Fig. 3b) negatively shifted to 931.9 and 951.9 eV compared to the standard Cu 2p peaks (932.6 and 952.56 eV). The binding energy of Co 2p peaks (Fig. 3c) showed a positive shift compared with the standard metallic Co peaks (778.3 and 793.2 eV), and the oxidized state Co also existed in the nanosurface of Pd59Cu30Co11 nanoalloys. The shifts of binding energy could be ascribed to the strong electronic effects between Pd, Cu, and Co elements through d-band hybridization upon alloying within the atomic range12,13,40,41.
In-depth composition and structural analyses of the Pd59Cu30Co11 nanoalloys. The X-ray photoelectron spectroscopy (XPS) spectra of the as-synthesized dendritic Pd59Cu30Co11 nanoalloys a Pd 3d region, b Cu 2p region, c Co 2p region (the red dash lines in pictures indicate the standard positions of Pd 3d, Cu 2p and Co 2p), d the X-ray absorption near edge structure (XANES) spectra for the Pd K-edge
ORR performance
The ORR performance of the dendritic Pd59Cu30Co11 nanoalloys have been investigated compared with commercial Pt/C, bimetallic Pd50Cu50 and Pd88Co12 and trimetallic Pd56Cu38Co6 and Pd62Cu16Co22 alloy nanodendrites (Supplementary Fig. 2, Supplementary Fig. 3, Supplementary Fig. 4, Supplementary Fig. 5, and Supplementary Table 1) obtained at the same synthetic method as the dendritic Pd59Cu30Co11 nanoalloys in an O2-saturated 0.1 mol L−1 (M) KOH solution with a scan rate of 10 mV s−1 and a rotation rate of 1600 pm at room temperature. The ORR polarization curves of these samples are shown in Fig. 4a. The half-wave potentials of the Pd50Cu50, Pd88Co12, Pd59Cu30Co11, Pd56Cu38Co6, Pd62Cu16Co22, and commercial Pt/C catalysts are 0.86, 0.90, 0.91, 0.90, 0.89, and 0.87 V, respectively, which exhibited that the Pd59Cu30Co11 nanoalloys had a greatly reduced ORR overpotential and indicated the superior activity. This was also confirmed by Tafel plots of these samples (Fig. 4a, inset). The positions of hydrogen adsorption/desorption peaks were often used to analyze the surface structure of electrocatalyst (Supplementary Fig. 6a). As can been seen, the hydrogen adsorption/desorption peaks on these samples located at different positon, which meant the surface structure and active site of these electrocatalysts were quite different and would display different ORR performance. The electrochemically active surface area (ECSA), calculated from the results of CO-stripping (Supplementary Fig. 7) of Pd50Cu50, Pd88Co12, Pd59Cu30Co11, Pd56Cu38Co6, and Pd62Cu16Co22 dendritic nanoalloys was around 37.9, 41.2, 58.7, 46.3, and 44.7 m2 g−1Pd, respectively. The ECSA of commercial Pt/C was 67.8 m2 g−1Pt. Figure 4b showed the specific activity (SA) and MA of ORR normalized by ECSA and Pd or Pt loading at 0.9 V versus a RHE using Koutecky–Levich equation, respectively. Among them, the Pd59Cu30Co11 nanoalloys showed the best SA and MA. The SA and MA on the Pd59Cu30Co11 nanoalloys were 0.90 mA cm−2 and 0.38 A mg−1Pd, respectively. The MA on Pd59Cu30Co11 nanoalloys was 3.45 times higher than commercial Pt/C catalysts (0.11 A mg−1Pt). Impressively, our Pd59Cu30Co11 nanoalloys had a competitively high MA in comparison with previously reported non-Pt metal nanocatalysts (Supplementary Table 2) that is also superior to the recently reported PdCuCo anisotropic structure (0.18 A mg−1Pd)40 and spherical PdCuCo (0.13 A mg−1Pd)43. Furthermore, at 0.875 and 0.8 V versus RHE (Fig. 4c, d), the Pd59Cu30Co11 nanoalloys exhibited the largest ORR SA (1.73 mA cm−2 at 0.875 V, 3.76 times that of commercial Pt/C; 10.20 mA cm−2 at 0.8 V, 3.98 times that of commercial Pt/C) and MA (1.01 A mg−1Pd at 0.875 V, 3.48 times that of commercial Pt/C; 5.98 A mg−1Pd at 0.8 V, 3.71 times that of commercial Pt/C). Furthermore, in order to determine the electron transfer number on Pd59Cu30Co11 nanoalloys, the ORR polarization curves of Pd59Cu30Co11 nanoalloys were investigated by rotating disk electrode (RDE) measurements at different rotation rates from 400 to 2500 rpm in 0.1 M KOH solution (Supplementary Fig. 8a). The Koutecky–Levich (K–L) plots for Pd59Cu30Co11 nanoalloys calculated at different potentials from 0.4 to 0.8 V were shown in Supplementary Fig. 8b. The slopes were parallel at different potentials, showing the first-order reaction kinetics and the electron transfer number was around four that indicated fully electrocatalytic reduction of oxygen into water on the Pd59Cu30Co11 nanoalloys. The alcohol (methanol or ethanol) tolerance of the dendritic Pd59Cu30Co11 nanoalloys was also investigated for ORR. Before testing ORR, the electrocatalytic activity toward methanol or ethanol of the dendritic Pd59Cu30Co11 nanoalloys and commercial Pt/C were performed using cyclic voltammetry in 0.1 M KOH + 0.1 M methanol/ethanol solution. As shown in Fig. 5a–c, typical methanol and EO peaks were observed at 0.88 and 0.87 V, respectively, on commercial Pt/C, while methanol oxidation peak was hardly generated on the dendritic Pd59Cu30Co11 nanoalloys and EO peak was only one-sixth of that on commercial Pt/C, which meant much lower electrocatalytic activity toward methanol and EO on the dendritic Pd59Cu30Co11 nanoalloys compared with commercial Pt/C. The ORR polarization curves (Fig. 5b–d) showed the commercial Pt/C had a weak methanol and ethanol tolerance capacity. The half-wave potentials of the commercial Pt/C are 0.707 and 0.619 V versus RHE in the presence of methanol and ethanol, respectively, and the half-wave potentials negatively shifted 0.163 and 0.251 V, respectively, compared with that (0.87 V versus RHE; Fig. 4a) without methanol and ethanol. However, the dendritic Pd59Cu30Co11 nanoalloys exhibited excellent methanol and ethanol tolerance capacity. The half-wave potential (0.91 V without methanol and ethanol; Fig. 4a) were barely affected by methanol (0.909 V versus RHE) and ethanol (0.907 V versus RHE). These results showed that the dendritic Pd59Cu30Co11 nanoalloys had much better antimethanol and ethanol interference ability than the commercial Pt/C.
Oxygen reduction reaction performance characterization. The oxygen reduction reaction (ORR) performance of Pt/C, Pd88Co12, Pd50Cu50, Pd56Cu38Co6, Pd59Cu30Co11, and Pd62Cu16Co22 catalysts. a The ORR polarization curves were recorded in O2-saturated 0.1 mol L−1 (M) potassium hydroxide (KOH) and the inset was the corresponding specific activity Tafel plots. Histograms of ORR specific activities and mass activities of the Pt/C, Pd88Co12, Pd50Cu50, Pd56Cu38Co6, Pd59Cu30Co11, and Pd62Cu16Co22 catalysts at 0.9 V (b), 0.875 V (c), and 0.8 V (d) versus reversible hydrogen electrode (RHE). e, f The ORR polarization curves for the Pd59Cu30Co11 and commercial Pt/C catalysts before and after cycles of accelerated durability tests (ADTs) (the inset was the corresponding mass activity at 0.90 V versus RHE). Error bars are ±s.d.
Methanol and ethanol tolerance tests. Cyclic voltammetry (CV) curves of the dendritic Pd59Cu30Co11 nanoalloys and the commercial Pt/C in N2-saturated 0.1 mol L−1 (M) potassium hydroxide (KOH) + 0.1 M methanol (CH3OH) (a) or ethanol (CH3CH2OH) (c) solution at 50 mV s−1. Oxygen reduction reaction (ORR) polarization curves of the dendritic Pd59Cu30Co11 nanoalloys and the commercial Pt/C in O2-saturated 0.1 M KOH + 0.1 M methanol (b) or ethanol (d) solution at 10 mV s−1 and rotation rate of 1600 rpm
The dendritic Pd59Cu30Co11 nanoalloys also presented long-term durability under the ORR reaction condition. The accelerated durability tests (ADTs) were used to evaluate ORR durability under a sweep rate of 100 mV s−1 between 0.53 and 1.03 V in O2-saturated 0.1 M KOH solution. After 5000, 10,000, and 15,000 sweeping cycles, the MA of the Pd59Cu30Co11 nanoalloy was 0.44, 0.40, and 0.26 A mg−1Pd, respectively, at 0.9 V versus RHE (Fig. 4e–f), which first experienced a rise and then a drop. This could be ascribed to the surface structure change under the ADTs and the same phenomenon was also observed about trimetallic PtPdAu alloys reported by Ding’s group54. However, the MA of Pt/C after 5000 and 10,000 sweeping cycles was 0.05 and 0.03 A mg−1Pt, respectively, at 0.9 V versus RHE. After 10,000 sweeping cycles, the Pt/C catalyst shows the 72.7% loss of MA, while the Pd59Cu30Co11 nanoalloys showed no loss of MA (0.40 A mg−1Pd) that was 13.3 times higher than Pt/C. And after 15,000 sweeping cycles, the MA (0.26 A mg−1Pd) of the Pd59Cu30Co11 nanoalloys is 2.36 times higher than initial MA (0.11 A mg−1Pt) of Pt/C and had a loss of 31.6% compared with the initial MA (0.38 A mg−1Pd). The above results exhibited that the Pd59Cu30Co11 nanoalloys had considerable enhancement with respect to durability compared with commercial Pt/C. After ADTs, we checked the morphologies of the Pd59Cu30Co11 nanoalloys using TEM (Supplementary Fig. 9). The TEM images showed the Pd59Cu30Co11 nanoalloys also maintained dendritic structure, however, after 15,000 sweeping cycles, the size of the small grains obviously increased due to the movement, aggregation and Ostwald ripening processes under the ADTs19, and we can clearly observe that some dendritic nanocrystals are hollow-like shapes, which caused by the electrochemical dealloying of Cu or Co (Supplementary Fig. 9), these were the reason why the catalytic activity decreased. After ADT cycles, the composition of Pd59Cu30Co11 nanoalloys changed (Supplementary Fig. 10). After 5000 cycles, the composition was 69.41 at% Pd, 29.29 at% Cu, and 1.30 at% Co. After 10,000 cycles, the composition was the 77.30 at% Pd, 21.78 at% Cu, and 0.92 at% Co, and after 15,000 cycles it was 83.68 at% Pd, 15.68 at% Cu and 0.64 at% Co. Moreover, the dendritic structure had stronger antiaggregation capacity due to their free-standing architectural feature than commercial Pt/C. For commercial Pt/C, after 10,000 cycles, the size of Pt particles obviously increased relative to initial size, namely, the size increased from sub-3.0 nm to around 10 nm (Supplementary Fig. 11), which led to the sharp fall of activity of commercial Pt/C.
Formic acid oxidation performance
The dendritic Pd59Cu30Co11 nanoalloys also exhibited excellent FAO activity. Figure 6a showed that the SA of the Pd50Cu50, Pd88Co12, Pd56Cu38Co6, Pd59Cu30Co11, Pd62Cu16Co22, commercial Pd black, and Pt/C were around 6.38, 2.94, 7.25, 9.06, 6.46, 2.24, and 0.56 mA cm−2, respectively. The MA (Fig. 6b) of the Pd50Cu50, Pd88Co12, Pd56Cu38Co6, Pd59Cu30Co11, Pd62Cu16Co22, commercial Pd black, and Pt/C was 2.42, 1.21, 3.36, 5.32, 2.89, 0.39 A mg−1Pd and 0.35 A mg−1pt, respectively. These values (Fig. 6c) showed that the as-synthesized Pd-based alloys displayed greatly enhanced activity compared with commercial Pd black and Pt/C. And the values of Pd59Cu30Co11 nanoalloys were the largest. The FAO SA and MA of the Pd59Cu30Co11 nanoalloys were 4.04 and 13.6 times higher than that of commercial Pd black, respectively, and 16.1 and 15.2 times higher than commercial Pt/C, respectively. Similar to the case of ORR, the Pd59Cu30Co11 nanoalloys display high MA relative to previously reported Pd-based electrocatalysts (Supplementary Table 3). Moreover, the stability of the as-synthesized PdCuCo nanoalloys was evaluated by current–time curves and showed better stability than Pd50Cu50, Pd88Co12, commercial Pd black and Pt/C (Fig. 6d).
Formic acid oxidation (FAO) characterization. Cyclic voltammetry (CV) curves (specific activity (a) and mass activity (b)) of the as-synthesized Pd50Cu50, Pd88Co12, Pd56Cu38Co6, Pd59Cu30Co11, Pd62Cu16Co22, commercial Pt/C, and Pd black with a scan rate of 50 mV s−1 in 0.1 mol L−1 (M) perchloric acid (HClO4) + 0.5 M formic acid (HCOOH) solution at room temperature. c Histograms of formic acid oxidation (FAO) specific activity and mass activity. Error bars are ±s.d. d Current–time curves of the as-synthesized Pd50Cu50, Pd88Co12, Pd56Cu38Co6, Pd59Cu30Co11, Pd62Cu16Co22, commercial Pt/C, and Pd black recorded at 0.25 V for 2500 s in 0.1 M HClO4 + 0.5 M HCOOH solution at room temperature
Discussion
The excellent electrocatalytic performance of the as-synthesized dendritic Pd59Cu30Co11 nanoalloys is mainly ascribed to the following reasons: (1) The special dendritic nanostructure of the Pd59Cu30Co11 nanoalloy can provide abundant defects, such as low-coordination number atoms (edges, corners, and steps), grain boundaries, lattice disorder, nanotwins, gap atoms, and vacancies, which have been confirmed to boost the catalytic performance in catalytic reactions4,19,43,47,48,49,50,51. (2) Synergistic surface effects between Pd, Cu, and Co elements are present in the topmost atomic layer and favor the removal of poisoning intermediates (such as CO adsorbed (COads) and OH adsorbed (OHads)) that are produced in the electrocatalytic reaction6,23,37,42. Moreover, metallic Cu and Co have been found to constitute sites for activating oxygen, water or small organic molecules and improve the activity and durability in FCs13,23,54,55,56,57,58,59,60,61,62. (3) Pd alloying with Cu and Co changes the surface electronic structure through ligand effect and downshifts the d-band center of Pd, which can tune the adsorption/desorption capacities of activating reactant molecules or intermediates and enhance the catalytic performance39,40,43. (4) Strain effects have also been shown to contribute to high catalytic performance7,10,12,60,61,62. The surface strain in dendritic Pd59Cu30Co11 nanoalloys may be attributed to the lattice mismatch triggered by the incorporation of smaller Cu and Co atoms into the Pd fcc lattice, small size of sub-5.0 nm grains that construct the dendritic structure and a boundary that originates from the mutual cross-linking of small grains59,60.
In conclusion, the defect-rich dendritic PdCuCo nanoalloys have been synthesized by a facile one-pot method to be developed as high-performance non-Pt nanocatalysts toward ORR and FAO. Among them, a Pd59Cu30Co11 nanoalloy shows the best catalytic performances. For ORR, the SA (0.90 mA cm−2 at 0.9 V and 1.73 mA cm−2 at 0.875 V versus RHE) and MA (0.38 A mg−1Pd at 0.9 V versus RHE and 1.01 A mg−1Pd at 0.875 V versus RHE) of the dendritic Pd59Cu30Co11 nanoalloy are 2.19 and 3.76 times and 3.45 and 3.48 times those of commercial Pt/C, respectively. The long-term durability has been evaluated through an ADT. After 10,000 cycles, the commercial Pt/C catalyst shows the 72.7% loss of MA, while the Pd59Cu30Co11 nanoalloy showed no loss of MA. After 15,000 cycles, the MA (0.26 A mg−1Pd) of the Pd59Cu30Co11 nanoalloy is 2.36 times higher than initial MA (0.11 A mg−1Pt) of Pt/C. For FAO, the SA and MA of the Pd59Cu30Co11 nanoalloy is 4.04 and 13.6 times higher, respectively, than commercial Pd black and 16.1 and 15.2 times higher, respectively, than commercial Pt/C. The current–time curves show that the Pd59Cu30Co11 nanoalloy exhibits better stability than commercial Pd black and Pt/C. And the dendritic Pd59Cu30Co11 nanoalloy has good methanol/ethanol tolerance ability. Thus, this work provides an example of the development of a new type of robust multifunctional, non-Pt catalysts for FCs.
Methods
Synthesis of dendritic Pd59Cu30Co11 nanoalloys
The dendritic Pd59Cu30Co11 nanoalloys were prepared by using precursors of Na2PdCl4, CuCl2, and CoCl2. Firstly, the 0.25 mL of 0.1 M Na2PdCl4, 0.125 mL of 0.1 M of 0.1 M of CuCl2, and 0.125 mL of 0.1 M of CoCl2 were added to 8.8 mL of ethylene glycol containing 0.050 g polyvinyl pyrrolidone (PVP-8000), and stirred for 1–2 min then the yellow clear solution was obtained. Secondly, the 0.2 mL ammonium hydroxide was added to the above solution, and stirred for 30 min. Then, the resulting clear solution was transferred to a 15-mL Teflon-lined stainless-steel autoclave. The oven was then heated at 150 °C for 8 h, then the Teflon-lined stainless-steel autoclave was taken out after it was cooled to room temperature. The products were separated several times by centrifugation and washing cycles at 10,000 rpm for 15 min with ethanol. The Pd59Cu30Co11 was redispersed in ethanol. By adjusting the ratio of Cu or Co precursors, the Pd50Cu50, Pd88Co12, Pd56Cu38Co6, and Pd62Cu16Co22 nanoalloys can be obtained under the same procedure. Further details are provided in the Supplementary Methods.
The ORR electrochemical measurement
For the ORR electrochemical measurement, a glassy carbon RDE (PINE, USA) was used. A Hg/HgO (0.1 M KOH) electrode and Pt black plate were used as reference and counter electrodes, respectively. The ORR was performed in the O2-saturated 0.1 M KOH solution at room temperature. Then, using a glassy carbon RDE at a rotation rate of 1600 rpm, the potential was scanned from 0.13 to 1.03 V (versus RHE) at a scan rate of 10 mV s−1 and were corrected for capacitive currents in N2 saturation. The kinetic current (ik) can be derived from the experimental data using the well-known Koutecky–Levich equation: (1/iK = 1/i − 1/iL) at 0.90 V versus RHE, where iL and i are the diffusion limiting current and measured current (0.90 V versus RHE) at kinetic-diffusion control region, respectively.
The tolerance test: A Hg/HgO (0.1 M KOH) electrode and Pt black plate were used as reference and counter electrodes, respectively. Then MOR or EOR tested with a scan rate of 50 mV s−1 at room temperature in 0.1 M KOH + 0.1 M CH3OH or 0.1 M KOH + 0.1 M CH3CH2OH, and the potential was scanned from 0.03 to 1.43 V (versus RHE), after this, using RDE at a rotation rate of 1600 rpm, and the potential was scanned from 0.13 to 1.03 V (versus RHE) at a scan rate of 10 mV s−1.
Data availability
All relevant data are available from the corresponding author upon reasonable request.
References
Steele, B. C. & Heinzel, A. Materials for fuel cell technologies. Nature 414, 345–352 (2001).
Othman, R., Dicks, A. L. & Zhu, Z. Nonprecious metal catalysts for the PEM fuel cell cathode. Int. J. Hydrogen Energy 37, 357–372 (2012).
Chen, A. & Holthindle, P. Platinum-based nanostructured materials: synthesis, properties, and applications. Chem. Rev. 110, 3767–3804 (2010).
Zhang, Z. et al. One-pot synthesis of highly anisotropic fivefold-twinned PtCu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation. Adv. Mater. 28, 8712–8717 (2016).
Zhang, N. et al. Superior bifunctional liquid fuel oxidation and oxygen reduction electrocatalysis enabled by PtNiPd core–shell nanowires. Adv. Mater. 29, 1603774 (2016).
Liu, T. et al. Monodispersed sub-5.0 nm PtCu nanoalloys as enhanced bifunctional electrocatalysts for oxygen reduction reaction and ethanol oxidation reaction. Nanoscale 9, 2963–2968 (2017).
Huang, X. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Casado-Rivera, E. et al. Electrocatalytic activity of ordered intermetallic phases for fuel cell applications. J. Am. Chem. Soc. 126, 4043–4049 (2004).
Wang, Y. et al. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 115, 3433–3467 (2015).
Bianchini, C. & Shen, P. K. ChemInform abstract: palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 109, 4183–4206 (2009).
Antolini, E. Palladium in fuel, cell catalysis. Energy Environ. Sci. 2, 915–931 (2009).
Suo, Y., Zhuang, L. & Lu, J. First-principles considerations in the design of Pd alloy catalysts for oxygen reduction. Angew. Chem. Int. Ed. 46, 2862–2864 (2007).
Maheswari, S., Karthikeyan, S., Murugan, P., Sridhar, P. & Pitchumani, S. Carbon-supported Pd–Co as cathode catalyst for APEMFCs and validation by DFT. Phys. Chem. Chem. Phys. 14, 9683–9695 (2012).
Holewinski, A., Idrobo, J. C. & Linic, S. High-performance Ag–Co alloy catalysts for electrochemical oxygen reduction. Nat. Chem. 6, 828–834 (2014).
Ahn, M. et al. Rhodium–tin binary nanoparticle-a strategy to develop an alternative electrocatalyst for oxygen reduction. ACS Catal. 7, 5796–5801 (2017).
Narayanamoorthy, B. et al. Enhanced intrinsic activity and stability of Au–Rh bimetallic nanostructures as a supportless cathode electrocatalyst for oxygen reduction in alkaline fuel cells. ACS Sustain. Chem. Eng. 4, 6480–6490 (2016).
Yan, Y. et al. Kinetically controlled growth of cubic and octahedral Rh–Pd alloy oxygen reduction electrocatalysts with high activity and durability. Nanoscale 7, 301–307 (2015).
Qi, Y. Wu et al. Facile synthesis of Rh–Pd alloy nanodendrites as highly active and durable electrocatalysts for oxygen reduction reaction. Nanoscale 6, 7012–7018 (2014).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Wang, X., Yang, J., Yi, H., Song, R. & Tang, Z. “Raisin bun”-like nanocomposites of palladium clusters and porphyrin for superior formic acid oxidation. Adv. Mater. 25, 2728–2732 (2013).
Feng, L. et al. Nanostructured palladium catalyst poisoning depressed by cobalt phosphide in the electrooxidation of formic acid for fuel cells. Nano Energy 30, 355–361 (2016).
Wang, C., Chen, D. P., Sang, X., Unocic, R. R. & Skrabalak, S. E. Size-dependent disorder–order transformation in the synthesis of monodisperse intermetallic PdCu nanocatalysts. ACS Nano 10, 6345–6353 (2016).
Zhang, L. et al. Pd–Cu bimetallic tripods: a mechanistic understanding of the synthesis and their enhanced electrocatalytic activity for formic acid oxidation. Adv. Funct. Mater. 24, 7520–7529 (2015).
Chen, Y. et al. High-yield synthesis of crystal-phase-heterostructured 4H/fcc Au@Pd core-shell nanorods for electrocatalytic ethanol oxidation. Adv. Mater. 29, 1701331 (2017).
Dai, Y. et al. Carbon monoxide-assisted synthesis of single-crystalline Pd tetrapod nanocrystals through hydride formation. J. Am. Chem. Soc. 134, 7073–7080 (2012).
Du, C., Chen, M., Wang, W. & Yin, G. Nanoporous PdNi alloy nanowires as highly active catalysts for the electrooxidation of formic acid. ACS Appl. Mater. Interfaces 3, 105–109 (2011).
Lu, Y., Jiang, Y., Gao, X., Wang, X. & Chen, W. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 136, 11687–11697 (2014).
Jiang, R. Tran, Mcclure, D. T. J. P. & Chu, D. A class of (Pd–Ni–P) electrocatalysts for the ethanol oxidation reaction in alkaline media. ACS Catal. 4, 2577–2586 (2014).
Jin, M., Zhang, H., Xie, Z. & Xia, Y. Palladium concave nanocubes with high-index facets and their enhanced catalytic properties. Angew. Chem. Int. Ed. 123, 7850–7854 (2011).
Zhou, Z., Kang, X., Song, Y. & Chen, S. Butylphenyl-functionalized palladium nanoparticles as effective catalysts for the electrooxidation of formic acid. Chem. Commun. 47, 6075–6077 (2011).
Huang, X. et al. Free-standing palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotech. 6, 28–32 (2011).
Chen, L. et al. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electrooxidation and oxygen reduction reactions. Adv. Funct. Mater. 21, 4364–4370 (2011).
Mazumder, V., MoreK. L. & SunS. et al. A facile synthesis of MPd (M = Co, Cu) nanoparticles and their catalysis for cormic acid oxidation. Nano Lett. 12, 1102–1106 (2012).
Ghosh, A., Chandran, P. & Ramaprabhu, S. Palladium–nitrogen coordinated cobalt alloy toward hydrogen oxidation and oxygen reduction reactions with high catalytic activity in renewable energy generations of proton exchange membrane fuel cell. Appl. Energy 208, 37–48 (2017).
Koenigsmann, C. & Wong, S. S. Tailoring chemical composition to achieve enhanced methanol oxidation reaction and methanol-tolerant oxygen reduction reaction performance in palladium-based nanowire systems. ACS Catal. 3, 2031–2040 (2013).
Guo, C. X., Zhang, L. Y., Miao, J., Zhang, J. & Li, C. M. DNA-functionalized graphene to guide growth of highly active Pd nanocrystals as efficient electrocatalyst for direct formic acid fuel cells. Adv. Energy Mater. 3, 167–171 (2013).
Zheng, Y. et al. Component-controlled synthesis and assembly of Cu–Pd nanocrystals on graphene for oxygen reduction reaction. ACS Appl. Mater. Interfaces 7, 5347–5357 (2015).
Zhang, Y. et al. Seedless growth of palladium nanocrystals with tunable structures: from tetrahedra to nanosheets. Nano Lett. 15, 7519–7525 (2015).
Yang, N. et al. Synthesis of ultrathin PdCu alloy nanosheets used as a highly efficient electrocatalyst for formic acid oxidation. Adv. Mater. 29, 1700769 (2017).
Zuo, Y. et al. Atomic vacancies control of Pd-based catalysts for enhanced electrochemical performance. Adv. Mater. 30, 1704171 (2018).
Liu, H., Koenigsmann, C., Adzic, R. R. & Wong, S. S. Probing ultrathin one-dimensional Pd–Ni nanostructures as oxygen reduction reaction catalysts. ACS Catal. 4, 2544–2555 (2014).
Liu, H., Zheng, Y., Wang, G. & Qiao, S. Z. A three-component nanocomposite with synergistic reactivity for oxygen reduction reaction in alkaline solution. Adv. Energy Mater. 5, 618–622 (2015).
Jiang, K. et al. Ordered PdCu-based nanoparticles as bifunctional oxygen reduction and ethanol-oxidation electrocatalysts. Angew. Chem. Int. Ed. 55, 9030–9035 (2016).
Wang, R. et al. One-pot solvothermal synthesis of PdCu nanocrystals with enhanced electrocatalytic activity toward glycerol oxidation and hydrogen evolution. Int. J. Hydrogen Energy 42, 6695–6704 (2017).
Dai, Q. & Tang, J. The optical and magnetic properties of CoO and Co nanocrystals prepared by a facile technique. Nanoscale 5, 7512–7519 (2013).
Xiao, W. et al. Optimizing the ORR activity of Pd-based nanocatalysts by tuning their strain and particle size. J. Mater. Chem. A 5, 9867–9872 (2017).
Zhao, W. et al. Highly active and durable Pt72Ru28 porous nanoalloy assembled with sub-4.0 nm particles for methanol oxidation. Adv. Energy Mater. 7, 1601593 (2017).
Zhang, N. et al. Oxide defect engineering enables to couple solar energy into oxygen activation. J. Am. Chem. Soc. 138, 8928–8935 (2016).
Lan, J., Wang, K., Yuan, Q. & Wang, X. Composition-controllable synthesis of defect-rich PtPdCu nanoalloys with hollow cavities as superior electrocatalysts for alcohol oxidation. Mater. Chem. Front. 1, 1217–1222 (2017).
Xu, G. et al. Morphological and interfacial control of platinum nanostructures for electrocatalytic oxygen reduction. ACS Catal. 6, 5260–5267 (2016).
Lim, B. et al. Pd–Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 324, 1302–1305 (2009).
Xue, Ha et al. Fabrication of PdCo bimetallic nanoparticles anchored on three-dimensional ordered N-doped porous carbon as an efficient catalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 8, 20766–20771 (2016).
Wang, A. et al. Palladium–cobalt nanotube arrays supported on carbon fiber cloth as high-performance flexible electrocatalysts for ethanol oxidation. Angew. Chem. Int. Ed. 54, 3669–3673 (2015).
Li, J. et al. Surface evolution of a Pt–Pd–Au electrocatalyst for stable oxygen reduction. Nat. Energy 2, 17111 (2017).
Fernández, J. L., Walsh, D. A. & Bard, A. J. Thermodynamic guidelines for the design of bimetallic catalysts for oxygen electroreduction and rapid screening by scanning electrochemical microscopy. M–Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 127, 357–365 (2005).
Chen, B., Cheng, D. & Zhu, J. Synthesis of PtCu nanowires in nonaqueous solvent with enhanced activity and stability for oxygen reduction reaction. J. Power Sources 267, 380–387 (2014).
Huang, S., Yang, N., Wang, S., Sun, Y. & Zhu, Y. Tuning the synthesis of platinum–copper nanoparticles with a hollow core and porous shell for the selective hydrogenation of furfural to furfuryl alcohol. Nanoscale 8, 14104–14108 (2014).
Chen, S., Su, H., Wang, Y., Wu, W. & Zeng, J. Size-controlled synthesis of platinum–copper hierarchical trigonal bipyramid nanoframes. Angew. Chem. Int. Ed. 54, 108–113 (2015).
Bu, L. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016).
Wu, J. et al. Surface lattice-engineered bimetallic nanoparticles and their catalytic properties. Chem. Soc. Rev. 41, 8066–8074 (2012).
Stamenkovic, V. R. et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6, 241–247 (2007).
Mavrikakis, M., Hammer, B. & Nørskov, J. K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 81, 2819–2822 (1998).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21571038, 21361005, 21431003, and 21521091), National Key R&D Program of China (2017YFA0700101 and 2016YFA0202801), the Open Fund of the Key Lab of Organic Optoelectronics & Molecular Engineering (Tsinghua University) and State Key Laboratory of Physical Chemistry of Solid Surfaces (201520). We also thank Professor Zhi-You Zhou (Xiamen University) for the help of ORR experiments and discussion.
Author information
Authors and Affiliations
Contributions
C.L. synthesized the catalysts, prepared the fuel cell test materials and performed electrocatalytic tests. C.L., B.N., T.H., S.Z., Y.L., and L.G. performed the structure characterizations (TEM, HRTEM, ICP, XPS, XRD, and EDS). Q.Y. and X.W. wrote and edited the manuscript and Supplementary information. All authors contributed to discussions about the results and the manuscript. The project was conceived and supervised by Q.Y. and X.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, C., Yuan, Q., Ni, B. et al. Dendritic defect-rich palladium–copper–cobalt nanoalloys as robust multifunctional non-platinum electrocatalysts for fuel cells. Nat Commun 9, 3702 (2018). https://doi.org/10.1038/s41467-018-06043-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-06043-1
This article is cited by
-
Unusual Sabatier principle on high entropy alloy catalysts for hydrogen evolution reactions
Nature Communications (2024)
-
Ultrathin curved PdNiRu nanosheets as bifunctional catalysts for oxygen reduction and ethylene glycol oxidation
Nano Research (2024)
-
Lamellar-assembled PdNi super-nanosheets as effective oxygen redox dual-electrocatalysts for rechargeable Zn-air batteries
Nano Research (2023)
-
Controlling the Size of Ag@Pd Catalysts to Boost Ethanol Oxidation
Journal of Electronic Materials (2023)
-
Surface cleaned CuxPdy alloy: Synthesis and their superior performances in hydrogen evolution reaction and biosensor
Nano Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.