Abstract
Although, a number of pathogenic mutations have been found for Alzheimer’s disease (AD), only one protective mutation has been identified so far in humans. Here we identify possible protective deletion mutations in the 3′-UTR of the amyloid precursor protein (App) gene in mice. We use an App knock-in mouse model carrying a humanized Aβ sequence and three AD mutations in the endogenous App gene. Genome editing of the model zygotes using multiple combinations of CRISPR/Cas9 tools produces genetically mosaic animals with various App 3′-UTR deletions. Depending on the editing efficiency, the 3′-UTR disruption mitigates the Aβ pathology development through transcriptional and translational regulation of APP expression. Notably, an App knock-in mouse with a 34-bp deletion in a 52-bp regulatory element adjacent to the stop codon shows a substantial reduction in Aβ pathology. Further functional characterization of the identified element should provide deeper understanding of the pathogenic mechanisms of AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, which is pathologically characterized by deposition of amyloid-beta peptide (Aβ), as well as hyperphosphorylated tau aggregation. Aβ is a 38–43 amino acid peptide produced from amyloid precursor protein (APP) through processing by two different proteases, β-secretase and γ-secretase. Genetic analyses of familial AD (FAD) provide fundamental evidence that Aβ accumulation plays a central role in AD pathogenesis1. Over 300 pathogenic mutations in the App gene or presenilin gene, encoding a catalytic subunit of γ-secretase, have been found to cause FAD through increased Aβ production and/or a change in the ratio of aggregation-prone Aβ species2. In contrast to the pathogenic mutations, only one protective mutation, which slows or prevents the onset or progression of AD pathology, has been identified to date. A previous human genomic study with a set of whole-genome sequence data from 1,795 Icelanders revealed that a missense mutation (p.A673T) on the App gene has a protective effect against the onset of AD, possibly via reducing the levels of amyloidogenic peptides3,4. However, subsequent replication studies failed to reproduce the protective effects of the variant due to its rarity5,6, underscoring the difficulty of exploring protective mutations by random screening based on a large human dataset. To facilitate the identification of protective mutations that occur infrequently or do not occur naturally in the human population, targeted screening based on relevant animal models of AD is necessary.
We recently established single-App knock-in model mice carrying a humanized Aβ sequence, as well as two or three clinically causative mutations in the endogenous murine App gene7,8. Generating these mice, we unexpectedly found that App knock-in mice lacking the last two introns (intron 16, 17) and 3′-UTR did not display Aβ deposition or deleterious effects at any ages due to a substantial reduction in APP expression at both the transcriptional and translational levels (Supplementary Fig. 1). The results led us to predict that these regions in the App gene affect Aβ accumulation via alteration of APP expression.
To test this theory, we here focused our attention on the App 3′-UTR because the sequence is highly conserved between mice and humans. We performed CRISPR/Cas9-mediated genome editing in App knock-in mouse zygotes in order to directly delete the App 3′-UTR. Subsequent quantitative Aβ measurements, as well as APP expression analyses in the 6-month-old edited model mice provided that deletion of the App 3′-UTR mitigated Aβ accumulation in the brain through the reduction of APP expression levels. Further experiments using another combination of genome editing tools enabled us to narrow down a possible responsible element in the App 3′-UTR. Our results suggest that targeted screening strategy based on relevant AD models would be useful for the identification of novel protective AD mutations.
Results
Generation of the App knock-in mice with App 3′-UTR deletion
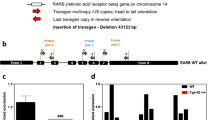
First, we explored whether disruption of the endogenous App 3′-UTR prevents Aβ accumulation in our AD mouse model. To this end, we deleted ~700-bp of the 891-bp App 3′-UTR in the mouse model zygotes using CRISPR/Cas9 technology (Fig. 1a, b)9,10. We selected an App knock-in mouse line (NL-G-F)7 as a suitable model for the following reasons: the genome editing technology cannot be applied to the conventional APP-Tg mice because they overexpress APP in a multicopy manner and because they fail to possess App non-coding regions that regulate transcription and translation of the gene. We designed a pair of CRISPR/Cas9 tools to delete the App locus between the two target sites (Fig. 1a). After checking the editing efficiency in culture cells, the CRISPR/Cas9 tools were injected into NL-G-F mouse zygotes (Supplementary Table 2). Two weeks after the birth of mice, we performed PCR-based genotyping using tail genomic DNA. Simultaneous genome editing revealed that cleaved fragments of the 3′-UTR could be detected in 14 founder mice (named as NL-G-F ΔUTR) out of 49 live-born pups (Supplementary Table 2). To exclude the possibility of heterogeneous genotypes among the tissues in the founder mice, as shown in previous studies11,12, we also performed PCR-based genotyping using NL-G-F ΔUTR mouse brains. Deleted fragments could be distinguished from parental band by agarose electrophoresis. Based on the intensity of the deleted fragments, we calculated the deletion efficiency of each sample (Fig. 1c; 0.2–74%). The deletion efficiency correlated well between the tail and brain samples. The exact sequences of deleted fragments were then identified using Sanger sequencing (Fig. 1d). We excluded one mouse (#12) with coding sequence deletion from the founder candidates. In parallel, we performed off-target analyses using Sanger sequencing on seven potential sites in the 14 NL-G-F ΔUTR, and could not find any off-target mutations in the founder mice (Supplementary Table 3).
Disruption of App 3′-UTR in NL-G-F knock-in mice. a Positions of targeting sgRNAs and detection primers used for CRISPR/Cas9-mediated deletion of App 3′-UTR. Red arrowheads indicate Cas9 cleavage sites within the sgRNA target sites. b Strategy for genome editing of homozygous NL-G-F mouse zygotes. c PCR-based genotyping results of NL-G-F ΔUTR mice. Genotyping was performed using mouse brain samples. In contrast to unedited NL-G-F, NL-G-F ΔUTR showed various deletion in the App 3′-UTR. The deletion efficiency was calculated as the relative ratio of the intensity of deleted fragments (parenthesis) to that of parental band (arrow). The efficiency of each NL-G-F ΔUTR mouse is represented on the bottom of each lane. d Exact sequences of cleaved fragments from four representative NL-G-F ΔUTR mice (#2, #7, #8, and #9 from Fig. 1c). PCR products from mouse brain DNA samples were subcloned into the pTAC-1 vector and individual clones were picked and sequenced
Deletion of App 3′-UTR mitigated Aβ pathology in the App knock-in mice
Next, we explored whether 3′-UTR deletion of App has inhibitory effects on Aβ accumulation. To this end, we performed immunohistochemical analyses of 6-month-old NL-G-F ΔUTR brains with anti-Aβ antibody, and found a drastic reduction of Aβ accumulation in several samples with highly disrupted App 3′-UTR (Fig. 2a, b). Notably, the 70% deleted sample displayed a significant reduction of Aβ accumulation which contrasted with the wide distribution of Aβ deposition found in the unedited 6-month-old NL-G-F mice (Fig. 2a). On the other hand, no clear differences in Aβ accumulation were observed between unedited NL-G-F mice and the 6% deleted sample (Fig. 2a). Consistent with these results, the deletion efficiency inversely correlated with Aβ accumulation in NL-G-F ΔUTR cortex (Fig. 2b), hippocampus and subcortical regions (Supplementary Fig. 2). We also checked the glial response in the NL-G-F ΔUTR cortex, and found a substantial reduction of the GFAP-positive area in highly edited NL-G-F ΔUTR cortex (Supplementary Fig. 3). To clarify the underlying mechanisms of the observed reduction in Aβ deposition, we performed real-time PCR analyses to quantify total RNA in samples from 6-month-old NL-G-F ΔUTR cortexes. GAPDH-normalized APP mRNA expression levels positively correlated with Aβ accumulation in NL-G-F ΔUTR mice (Supplementary Fig. 4). We also validated the correlation at the protein level: APP protein expression levels significantly correlated with Aβ accumulation in NL-G-F ΔUTR mice (Fig. 2c); although, the intensity between unedited samples were somewhat varied possibly due to the presence of fractionation process (Supplementary Fig. 5). Furthermore, we performed quantitative Aβ ELISA, and confirmed that the deletion efficiency inversely correlated with both Aβ40 and Aβ42 levels in NL-G-F ΔUTR cortex (Fig. 2d–g). These results provide the first evidence that CRISPR/Cas9-mediated deletion of the App 3′-UTR in AD model mouse zygotes mitigates Aβ pathology development via reduction of APP mRNA and protein expression levels.
Prevention of Aβ accumulation in 6-month-old App 3′-UTR deleted NL-G-F mice. a Aβ pathology in 6-month-old NL-G-F ΔUTR mouse brains, as well as unedited control NL-G-F brains. Scale bar: 1 mm. b Negative correlation between Aβ accumulation and genome editing efficiency in NL-G-F ΔUTR mouse brains (n = 13). c Positive correlation between normalized APP protein expression and genome editing efficiency in NL-G-F ΔUTR mouse brains (n = 13). Western blotting was performed using an antibody against N terminus of APP protein. d–g Negative correlation between normalized Aβ levels and deletion efficiency in 6-month-old NL-G-F ΔUTR mouse brains (n = 13). Aβ40 and Aβ42 levels in both the Tris-HCl-buffered saline (TS) and GuHCl fractions were quantified by ELISA. h Strategy of CRISPR/Cas9-mediated NL-G-F Δ400. Positions of targeting sgRNAs are shown by scissors. i Negative correlation between Aβ levels and deletion efficiency in female NL-G-F Δ400 mouse brains (n = 5). j Positive correlation between normalized APP protein expression and genome editing efficiency in NL-G-F Δ400 mouse brains (n = 11)
Further, we injected another combination of CRISPR tools, which targets ~400-bp of App 3′-UTR, into NL-G-F mouse zygotes (Fig. 2h). Simultaneous genome editing revealed that deleted fragments of the 3′-UTR were detected in 14 founder mice (named NL-G-F Δ400) out of 62 live-born pups (Supplementary Fig. 6 and Supplementary Table 2). We excluded three mice with relatively large insertions or inverted sequence from the founder candidates. Consistent with the results for the NL-G-F ΔUTR mice, subsequent ELISA of 6-month-old NL-G-F Δ400 cortexes showed a clear inverse correlation between Aβ levels and the deletion efficiencies (Fig. 2i). We also found the positive correlation between Aβ accumulation and APP expression (Fig. 2j). The clear negative correlation between APP mRNA expression and genome editing efficiency in wild-type Δ400 cortex further confirmed the presence of regulatory elements of APP expression (Supplementary Fig. 7).
52-bp element is possible sequence counteracting Aβ pathology
Previous in vitro and cell culture experiments identified several regulatory elements within the corresponding 400-bp region of the human homologue13,14. Of them, a 52-bp element adjacent to a stop codon on the last exon of the App gene seemed to play an important role in APP mRNA stabilization. Importantly, the 52-bp element is highly conserved between mice and humans (Fig. 3a), although it remains unclear whether the 52-bp element has the same role in mouse brain in vivo. To narrow down the responsible elements on the App 3′-UTR, we injected another combination of CRISPR tools to cut out an ~100-bp region, including the 52-bp element, into NL-G-F mouse zygotes (Fig. 3b). Two weeks after birth of the mice, we performed PCR-based genotyping using tail genomic DNA, and identified the founder candidates with deleted fragments (Supplementary Table 2). Based on the Sanger sequencing results, we excluded two mice with relatively large insertions from the founder candidates. We also performed PCR-based genotyping with the brain genomic DNA, and calculated the deletion efficiency (Supplementary Fig. 8). To determine the deleted sequences of nine founder mice (named NL-G-F Δ100), we performed TA cloning with the PCR-amplified DNA fragments derived from each founder mouse brain. We randomly picked up ~10 colonies for each founder, and then performed colony sequencing. Expectedly, ~100-bp region of App 3′-UTR were deleted in seven out of nine founders (Supplementary Fig. 9). Immunohistochemical analyses with anti-Aβ antibody were performed using tissue from 6-month-old NL-G-F Δ100 mice and un-injected control NL-G-F mice, and their Aβ pathology was compared. Among the nine founders, one founder (#3) showed a clear reduction in Aβ plaque area (Fig. 3c). Subsequent colony sequencing of the relevant brain sample revealed that a bi-allelic 34-bp deletion within the 52-bp element had occurred in that founder (Fig. 3d). To validate the immunohistochemistry result, we used an ELISA assay to quantitatively detect the alteration of Aβ levels in the CRISPR-injected founder mice, and found a substantial reduction of cortex Aβ levels in founder #3 (Fig. 3e). Importantly, the bi-allelic 34-bp deletion resulted in a substantial but not complete reduction in APP expression (Fig. 3f, g), possibly excluding unintended side effects derived from complete APP deficiency15,16 because heterozygous and homozygous App knock-out mice exhibit no and little abnormality, respectively, due to the functional redundancy of the APP family17. These results strongly support the previous results obtained from human cell culture experiments, and suggest that the 34-bp deletion could be used as a protective mutation to counteract the pathological changes in FAD. Although several other NL-G-F Δ100 mice also showed decreased Aβ levels (Supplementary Fig. 10) with somewhat reduced APP expression (Supplementary Fig. 11), no negative correlation was observed between the deletion efficiency and Aβ levels or APP expression levels. However, it was difficult to conclude that the 100-bp disruption did not affect Aβ accumulation through the regulation of APP expression due to the relatively low-deletion efficiency (10–30%) (Supplementary Fig. 8), as well as the genetic mosaicism of the NL-G-F Δ100 mice (Supplementary Fig. 9). Further investigations using the heterozygous or homozygous mutant offspring with various deletion mutations could lead to the in vivo identification of the responsible elements.
In vivo exploration of the regulatory elements on App 3′-UTR. a Sequence conservation of 52-bp regulatory element between the mouse and human. b Strategy for in vivo disruption of the 52-bp element by CRISPR/Cas9. Positions of targeting sgRNAs are shown by scissors. c Aβ pathology in 6-month-old NL-G-F Δ100, as well as unedited control NL-G-F brains. Scale bar: 1 mm. d Sequence analysis of founder #3 with bi-allelic 34-bp deletion. The fractions on the right indicate the mutant reads number out of total reads number. e Reduced Aβ levels in a 6-month-old NL-G-F Δ100 mouse brain (#3). Three unedited NL-G-F samples were used for normalization. The unedited control data represent mean ± SD. f A representative APP western blot image for 6-month-old NL-G-F Δ100 mouse brains. g Reduced APP expression at transcriptional and translational levels in a NL-G-F Δ100 mouse brain (#3). Three unedited NL-G-F samples were used for normalization. The unedited control data represent mean ± SD
Discussion
With the rapid progress of sequencing technologies, genetic variations affecting the risk of AD has been directly explored in the human population. Although such random screening using the large human dataset has been successful with the identification of some critical genetic variants3,18,19, it is still challenging to extract lower frequency mutations. In fact, the allele frequencies of the identified variants are much higher than those of all genetic variants within the 52-bp element on App 3′-UTR in the general population from the Exome Aggregation Consortium (ExAC)20 and the Genome Aggregation Database (gnomAD) (Supplementary Fig. 12). Moreover, the conventional screening strategy is not applicable to the identification of variants that do not occur naturally in the human population. To complement the random screening based on a large human dataset, we utilized genome editing-based targeted screening focusing on the App 3′-UTR for exploration of protective deletion mutations. As shown in this study, combination of CRISPR/Cas9 technology with the relevant App knock-in mouse model might allow direct evaluation of the protective effects derived from deletion mutations on Aβ deposition.
A large number of clinical trials have already been undertaken or are ongoing, but to date, no effective medications are available for AD. Given that AD affects ~34 million individuals worldwide21, there is an urgent need to develop strategies that take a new approach to preventing AD. Apart from AD, several loss-of-function variants, which occur naturally in the human population, have been identified as protective mutations against incurable conditions: e.g., a 32-bp deletion mutation on the CCR5 coding region for HIV infection22, and a 13-bp deletion mutation on the HBG1 promoter for hemoglobinopathies23. Utilizing this knowledge of protective mutations, ex vivo or in vivo genome editing strategies targeting the endogenous genes are becoming possible treatment options for these diseases24,25,26. The identification of a deletion mutation on CCR5 has also resulted in the development of an effective antiretroviral antagonist-based therapeutic approach27,28. In a similar fashion, the identification of novel protective mutations could provide new opportunities for the development of a novel therapeutic application for AD.
Methods
Animal use and care
We used 6-month-old CRISPR-injected App knock-in mice7, as well as unedited control model mice (C57BL/6). Both male and female mice were used. All experimental procedures were conducted in accordance with standard guidelines for animal experiments of RIKEN Center for Brain Science.
Preparation of Cas9 and sgRNAs
We constructed the plasmid vector px330 (Addgene #42230) expressing single-guide RNA (sgRNA) as well as Cas9. The px330 plasmid9 was a gift from Feng Zhang, and the sgRNA was designed in silico via the CRISPR Design tool29 or sgRNA designer30. For in vitro synthesis of Cas9 mRNA, plasmid vector pCAG-T3-hCAS-pA (Addgene #48625) was linearized by SphI, then transcribed with T3 RNA polymerase (Promega, Madison, WI, USA) in the presence of Ribo m7G Cap Analog (Promega) as described previously31. pCAG-T3-hCAS-pA was a gift from Wataru Fujii & Kunihiko Naito. sgRNAs were synthesized in vitro as previously described32 using a MEGAshortscript T7 kit (Thermo Fisher Scientific, Waltham, MA, USA). Synthesized RNAs were purified using a MEGAclear kit (Thermo Fisher Scientific). All sequences of oligonucleotides for sgRNA synthesis are listed in Supplementary Table 1.
Microinjections into mouse embryos
The Cas9 mRNA and sgRNAs were injected into C57BL/6 mouse zygotes or NL-G-F mouse zygotes using a microinjection system under standard conditions (as detailed in Supplementary Table 2). The injected zygotes were cultured in culture medium at 37 °C in 5% CO2 up to two-cell embryos, and then the embryos were transferred into the oviduct of the recipient mice.
Genotyping analysis
Genomic DNA was extracted from tail biopsies using a standard ethanol precipitation method. Genomic DNA from brain samples was isolated using TRIzol (Ambion) reagent according to the manufacturer’s instructions. Genomic DNA was amplified using a specific primer set to identify founder mice with App 3′-UTR deletion (Supplementary Table 1). PCR products that were ~100 bp or smaller than parental band were defined as deleted fragments. Intensity of the deleted band and intact parental band was quantified using ImageJ, and the deletion efficiency of each sample was represented as the ratio of the deleted band intensity to total band intensity (i.e., deleted band intensity plus parental band intensity). Sequencing analysis was used to further determine the mouse genotype.
Off-target analysis
Potential off-target sites with up to 3 bp mismatches were identified in silico using COSMID33. Target fragments on the off-target site were amplified using specific primer sets (Supplementary Table 3), and then sequencing analyses were carried out to detect mutations.
Immunohistochemical analysis
Immunohistochemical staining against Aβ (N1D, 1:200)34 and glial fibrillary acidic protein (GFAP, 1:100; MAB3402; Millipore, Darmstadt, Germany) was performed in 6-month-old NL-G-F mice. Paraffin-embedded mouse brain sections were immunostained as previously described7. Briefly, after deparaffinization, antigen retrieval was performed by autoclave treatment (121 °C for 5 min). The sections were rinsed several times, blocked for 30 min, and then incubated with primary antibody in blocking solution overnight. A biotinylated secondary antibody and tyramide signal amplification (PerkinElmer, Waltham, MA, USA) were used for detection of amyloid pathology. A secondary antibody conjugated with Alexa Fluor 488 (1:500; Invitrogen, Carlsbad, CA) was used for detection of astrocytosis. Photographic data were captured using a NanoZoomer Digital Pathology C9600 (Hamamatsu Photonics, Hamamatsu, Japan). Immunoreactive areas were quantified using Tissue Studio software (Definiens, Cambridge, MA, USA). To reduce the effect of variation between tissue sections, we used the average of data from 3 to 5 sections per mouse.
Quantitative RT-PCR
Total RNA was isolated from mouse cortex using TRIzol reagent (Ambion) according to the manufacturer’s instructions. Total RNA (1 µg aliquots) was converted to cDNA by ReverTra Ace (Toyobo, Osaka, Japan) and oligo (dT) primer. The resultant cDNA was diluted to 1:50 and the diluted solution was used as a sample. The quantitative PCR was performed using real-time PCR master mix (Toyobo) and specific TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) for mouse APP (Mm01344172_m1) and GAPDH (Mm99999915_g1). The ABI 7900HT system (Applied Biosystems) was used for amplification and detection. Relative expression was calculated by using the comparative cycle threshold (CT) method. The relative mRNA levels were normalized to endogenous ACTB or GAPDH mRNA expression for each sample. The CT value was obtained from the amplification plot with the aid of SDS software.
Northern blotting analysis
Total RNA was isolated from mouse cortex using and Micro-Fast Track 2.0 kit (invitrogen). RNA samples (6 μg) were separated in 1% formaldehyde agarose gels and transferred to nylon membranes. The membranes were reacted with isotope labeled cDNA fragment generated by PCR. The membranes were reprobed with an β-actin cDNA. cDNA fragments were amplified using specific primer sets: APP forward 5′-ATGTGCAGAATGGAAAGT-3′ and APP reverse 5′-CAGCATACAAACTCTACC-3′, β-actin forward 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ and β-actin reverse 5′-CTTAGAAGCATTTGCGGTGCACGATG-3′.
Western blotting analysis
Mouse cortexes were homogenized in 50 mM Tris-HCl (pH 7.4) containing protease inhibitor cocktail complete mini (Roche Diagnostics) (homogenizing buffer). After centrifugation at 1200×g, the supernatant was centrifuged at 200,000×g for 20 min at 4 °C. The pellet fraction was collected and then solubilized with homogenizing buffer containing 1% Triton X-100 for 60 min on ice and centrifuged again at 200,000×g for 20 min at 4 °C. The supernatants served as protein samples for further analyses to detect APP protein expression. Protein samples (20 μg) were separated by 10% SDS-PAGE, followed by transfer to PVDF membranes. After incubating with 2% ECL blocking reagent, the membranes were incubated with the N-terminal APP antibody (1:2000; 22C11, Millipore) or the C-terminal APP antibody (1:10,000; SIGMA, St. Louis, MO, USA) at 4 °C overnight. Then, the membranes were repeatedly washed and incubated with horseradish peroxidase-conjugated IgG secondary antibodies (1:5000; GE healthcare, Little Chalfont, UK). The band intensity was determined with a densitometer (LAS4000, Fujifilm). Each set of experiments was repeated twice. Results were the mean of the multiple experiments. APP expression levels in CRISPR-injected NL-G-F mouse brains were normalized with those in three or four unedited NL-G-F mouse brains. Normalized APP expression levels correlated well between the experiments with two distinct APP antibodies (Supplementary Fig. 13). Some of the membranes were reprobed with the Syntaxin 1B rabbit polyclonal antibody (1:10,000; Synaptic Systems, Goettingen, Germany).
Enzyme-linked immunosorbent assay (ELISA)
The quantitation of Aβ levels in 6-month-old NL-G-F mice was performed as previously described35. Briefly, mouse cortexes were homogenized in 50 mM Tris-HCl (pH 7.6) containing 150 mM NaCl and the protease inhibitor cocktail (TBS) with a Teflon-glass homogenizer. After centrifugation at 200,000×g for 20 min at 4 °C, the supernatant was collected and defined as the soluble fraction (TS fraction). The pellet was dissolved in 6 M guanidine–HCl buffer containing the protease inhibitor cocktail. The solubilized pellet was centrifuged at 200,000×g for 20 min at room temperature and then used as the insoluble fraction (GuHCl fraction). The amounts of Aβ40 and Aβ42 in each fraction were determined by Aβ ELISA kit (Wako Pure Chemicals, Osaka, Japan) according to the manufacturer’s instructions. Results were the mean of duplicate determinations. Because all Aβ peptides in NL-G-F mouse brains contain the Arctic mutation within the peptide sequence, the levels of Aβ40 and Aβ42 were quantified based on standard curves using synthetic human Arctic Aβ peptides (Peptide Institute, Osaka, Japan)7. Aβ levels in CRISPR-injected NL-G-F mouse brains were normalized with those in three or four unedited NL-G-F mouse brains.
Statistical analyses
The data were first analyzed for normal distribution. When normally distributed, the variables were statistically analyzed using two-tailed Pearson’s correlation coefficient. If the data did not pass normality testing, Spearman’s correlation coefficient was used. All analyses were completed with Statcel 3 (add-in software for Excel, Microsoft), and values of P < 0.05 were considered to be statistically significant.
Data availability
The data related to the findings of this study are available from the corresponding author on request.
References
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016).
Rosenberg, R. N., Lambracht-Washington, D., Yu, G. & Xia, W. Genomics of Alzheimer disease: a review. JAMA Neurol. 73, 867–874 (2016).
Jonsson, T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 (2012).
Kokawa, A. et al. The A673T mutation in the amyloid precursor protein reduces the production of beta-amyloid protein from its beta-carboxyl terminal fragment in cells. Acta Neuropathol. Commun. 3, 66 (2015).
Ting, S. K. et al. Absence of A673T amyloid-beta precursor protein variant in Alzheimer’s disease and other neurological diseases. Neurobiol. Aging 34, 2441.e2447–2448 (2013).
Wang, L. S. et al. Rarity of the Alzheimer disease-protective APP A673T variant in the United States. JAMA Neurol. 72, 209–216 (2015).
Saito, T. et al. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 17, 661–663 (2014).
Sasaguri, H. et al. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 36, 2473–2487 (2017).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Parikh, B. A., Beckman, D. L., Patel, S. J., White, J. M. & Yokoyama, W. M. Detailed phenotypic and molecular analyses of genetically modified mice generated by CRISPR-Cas9-mediated editing. PLoS ONE 10, e0116484 (2015).
Yen, S. T. et al. Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev. Biol. 393, 3–9 (2014).
Broytman, O., Westmark, P. R., Gurel, Z. & Malter, J. S. Rck/p54 interacts with APP mRNA as part of a multi-protein complex and enhances APP mRNA and protein expression in neuronal cell lines. Neurobiol. Aging 30, 1962–1974 (2009).
Rajagopalan, L. E., Westmark, C. J., Jarzembowski, J. A. & Malter, J. S. hnRNP C increases amyloid precursor protein (APP) production by stabilizing APP mRNA. Nucleic Acids Res. 26, 3418–3423 (1998).
Tremml, P., Lipp, H. P., Muller, U., Ricceri, L. & Wolfer, D. P. Neurobehavioral development, adult openfield exploration and swimming navigation learning in mice with a modified beta-amyloid precursor protein gene. Behav. Brain. Res. 95, 65–76 (1998).
Zheng, H. et al. Beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 81, 525–531 (1995).
Heber, S. et al. Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J. Neurosci. 20, 7951–7963 (2000).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2013).
Steinberg, S. et al. Loss-of-function variants in ABCA7 confer risk of Alzheimer’s disease. Nat. Genet. 47, 445–447 (2015).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Barnes, D. E. & Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828 (2011).
Samson, M. et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996).
Gilman, J. G. et al. Distal CCAAT box deletion in the A gamma globin gene of two black adolescents with elevated fetal A gamma globin. Nucleic Acids Res. 16, 10635–10642 (1988).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910 (2014).
Traxler, E. A. et al. A genome-editing strategy to treat beta-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 22, 987–990 (2016).
Fatkenheuer, G. et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 11, 1170–1172 (2005).
Gulick, R. M. et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N. Engl. J. Med. 359, 1429–1441 (2008).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Doench, J. G. et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267 (2014).
Fujii, W., Kawasaki, K., Sugiura, K. & Naito, K. Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease. Nucleic Acids Res. 41, e187 (2013).
Yang, H., Wang, H. & Jaenisch, R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9, 1956–1968 (2014).
Cradick, T. J., Qiu, P., Lee, C. M., Fine, E. J. & Bao, G. COSMID: a Web-based tool for identifying and validating CRISPR/Cas off-target sites. Mol. Ther. Nucleic Acids 3, e214 (2014).
Saido, T. C., Yamao-Harigaya, W., Iwatsubo, T. & Kawashima, S. Amino- and carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neurosci. Lett. 215, 173–176 (1996).
Iwata, N. et al. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J. Neurosci. 24, 991–998 (2004).
Acknowledgements
We thank Yukiko Nagai for secretarial assistance, Charles Yokoyama for comments on the manuscript, and Saido laboratory members for assistance. We are grateful to the RIKEN Center for Brain Science Research Resources Division for carrying out the injections of the CRISPR/Cas9 system into mouse zygotes, as well as for technical help with DNA sequencing analyses. This work was supported financially by an Incentive Research Grant from RIKEN (K.N. and F.O.-U.), the Special Postdoctoral Researchers Program from RIKEN (K.N. and S.H.), RIKEN Center for Brain Science (T.C.S.), Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and Development (T.C.S.), and the Aging Project of RIKEN (T.C.S.).
Author information
Authors and Affiliations
Contributions
K.N. and T.C.S. conceived the study. K.N. and T.S. designed the experiments. K.N., M.T., Y.M., F.O.-U. and K.S. performed the experiments. K.N. analyzed the data. S.H. and T.S. provided biological specimens and technical assistance. K.N. and T.C.S. wrote and edited the manuscript. All authors provided feedback and agreed on the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
T.S., S.H., Y.M., and T.C.S. serve as advisor, research scientist, director, and CEO, respectively, for RIKEN BIO Co. Ltd., a RIKEN venture based at RIKEN Center for Brain Science, Central Building, Wako, Japan.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagata, K., Takahashi, M., Matsuba, Y. et al. Generation of App knock-in mice reveals deletion mutations protective against Alzheimer’s disease-like pathology. Nat Commun 9, 1800 (2018). https://doi.org/10.1038/s41467-018-04238-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-04238-0
This article is cited by
-
A society-wide conversation is needed about germline genome editing using CRISPR
Nature Medicine (2024)
-
Advances in CRISPR therapeutics
Nature Reviews Nephrology (2023)
-
Advanced molecular therapies for neurological diseases: focus on stroke, alzheimer's disease, and parkinson's disease
Neurological Sciences (2023)
-
CRISPR: a tool with potential for genomic reprogramming in neurological disorders
Molecular Biology Reports (2023)
-
Exosomes for CRISPR-Cas9 Delivery: The Cutting Edge in Genome Editing
Molecular Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.