Abstract
During helminth infection and allergic asthma, naive CD4+ T-cells differentiate into cytokine-producing Type-2 helper (Th2) cells that resolve the infection or induce asthma-associated pathology. Mechanisms regulating the Th2 differentiation in vivo remain poorly understood. Here we report that mice lacking Bcl11b in mature T-cells have a diminished capacity to mount Th2 responses during helminth infection and allergic asthma, showing reduced Th2 cytokines and Gata3, and elevated Runx3. We provide evidence that Bcl11b is required to maintain chromatin accessibility at Th2-cytokine promoters and locus-control regions, and binds the Il4 HS IV silencer, reducing its accessibility. Bcl11b also binds Gata3-intronic and downstream-noncoding sites, sustaining the Gata3 expression. In addition, Bcl11b binds and deactivates upstream enhancers at Runx3 locus, restricting the Runx3 expression and its availability to act at the Il4 HS IV silencer. Thus, our results establish novel roles for Bcl11b in the regulatory loop that licenses Th2 program in vivo.
Similar content being viewed by others
Introduction
The molecular pathways dictating effector cell differentiation from naive CD4+ T-cells are controlled by transcription factors that regulate the expression of lineage-specific genes. Several of these transcription factors act as pioneers and initiate large scale changes in genetic programs by altering the chromatin landscape to create accessible regions at promoters, enhancers, and locus-control regions (LCRs)1. Type-2 T-helper (Th2) cells are formed following the activation of naive CD4+ T-cells in the presence of IL-4, and are critical in helminth infections and allergic diseases including asthma2. IL-4 is known to activate the signal transducer and activator of transcription 6 (STAT6)3, which in turn induces expression of GATA3, a potent pioneer transcription factor that acts at the Th2-LCR, and Th2-cytokine promoters4. By enhancing the expression of IL-4, GATA3 enforces a positive feedback loop that stabilizes the Th2 lineage2. However, compared to the other T-helper effector lineages, our understanding of the mechanisms behind Th2 differentiation in vivo is incomplete. The role of the canonical IL-4/STAT6 pathway, which has been used in in vitro CD4+ T-cell polarization for many years, generated conflicting reports in vivo5, and STAT6-independent mechanisms of Th2 differentiation have been identified4.
The Th2 cytokine locus, which contains the Il4, Il5, and Il13 genes, is under the control of an LCR located within the 3′ end of the Rad50 gene6. In vivo-deletion studies have shown that mice lacking the Th2 LCR have significantly impaired Th2 cytokine secretion and do not develop severe asthma7. The Th2 LCR contains four functionally distinct DNase hypersensitive sites (HSs), of which three are Th2 specific: Rad50 (R) HS IV, V, and VII. RHS VII has been shown to be critical in forming a poised-chromatin structure, which initiates the long-range interactions between the LCR and the Th2-cytokine promoters8. RHS IV needs to have a transcriptionally active configuration promoted by SATB19, while RHS V is needed to enhance the Il4 transcription through interactions with the Il4 promoter mediated by GATA3, OCT-1, and ETS-110. In addition to the LCR, Th2 differentiation is controlled by a conserved silencer, downstream of the Il4 gene at the HS IV11. During Th1 differentiation, the transcription factor Runx3 associates with the Il4 HS IV silencer to block Il4 transcription12,13. In addition, Runx3 attenuates the activity of GATA3 through direct interaction14.
Bcl11b functions both as a transcriptional repressor, when associated with the Nucleosome Remodeling and Deacetylase (Mi-2/NuRD) complex15,16,17, and as a transcriptional activator, when associated with the p300 histone acetyl transferase18. Bcl11b is expressed in thymocytes starting at the DN2 stage, playing major roles in the commitment to T-cell lineage. It further controls the beta and positive selection of thymoctes19,20,21,22,23 and is critical for the development of T-regulatory cells and iNKT cells24,25,26 (and reviewed in ref. 27). Bcl11b also controls cytotoxic T-cell function in bacterial and viral infections28,29, and is expressed in naive and effector CD4+ T-cells23,28. Bcl11b blocks GATA3 and IL4 in pathogenic Th17 cells during experimental autoimmune encephalomyelitis (EAE), thus controlling the plasticity of Th17 cells30. Bcl11b is also critical for type-2 innate lymphoid cell (ILC2s) development31,32, maintenance of their program and identity, as well as for the repression of type-3 ILC program in ILC2s33.
Here, we ascertain a new role for Bcl11b in the network of transcription factors that control differentiation of the Th2 lineage in vivo. We identified major defects in the capacity of Bcl11b-deficient T-helper cells to differentiate into Th2 cells in vivo, causing diminished responses to helminth infection and reduced severity of asthma. By evaluating the genome-wide binding of Bcl11b and comparing the changes in the transcriptome and chromatin accessibility, we established that Bcl11b-deficient T-helper cells fail to upregulate GATA3, express Runx3, and have enhanced chromatin accessibility at the Il4 HS IV silencer, but reduced accessibility at Th2-cytokine LCR and Th2-cytokine promoters. We position Bcl11b as a direct negative regulator of Runx3-gene expression, by binding and blocking two upstream enhancers in Th2 cells and naive CD4+ T-cells. In addition, we found the Bcl11b binding to conserved cis-regulatory sites located in the introns and downstream to the Gata3 locus. Thus, the reduction in GATA3, combined with increased Runx3 activity at the accessible Il4 HS IV silencer and diminished IL-4 expression in the absence of Bcl11b, resulted in diminished chromatin opening at the Th2 LCR, and at the Il13 and Il5 promoters, followed by reduced Th2 cytokine expression. This cements Bcl11b as an important transcription factor in Th2 lineage licensing. In addition, Bcl11b-deficient Th2 cells showed abnormal expression of alternate-lineage genes, including those of Th17, Th1, and innate cells.
Results
Removal of Bcl11b in T-cells attenuates allergic asthma
Our previous studies have shown that in mice induced with EAE, the absence of Bcl11b in mature T-cells caused increased plasticity of Th17 cells by acquisition of GATA3 and IL-430. However, we found that Bcl11b plays a critical role in ILC2s, promoting their program and preserving their identity33. Given these results, we investigated the role of Bcl11b during a house dust mite (HDM)-induced asthma, a Th2-mediated disease. For these studies, we used Bcl11bfl/fl dLck-iCre mice, whose Cre-recombinase is under the distal Lck promoter, which becomes active only in mature T-cells34. We have previously shown that this late-deleting-Cre system removes Bcl11b in ~80% of the mature CD4+ T-cells, and does not cause the removal of Bcl11b in DP and single-positive thymocytes, allowing T-cells to reach normal maturity28.
Following sensitization and challenge with HDM extract derived from Dermatophagoides pteronyssinus (Supplementary Figure 1a), we found that Bcl11bfl/fl dLck-iCre mice developed less severe lung pathology than wild-type (WT) mice, with substantially reduced leukocyte infiltration (Fig. 1a). Eosinophils, which are recruited to the lung during HDM-induced asthma, were present in significantly lower numbers in Bcl11bfl/fl dLck-iCre mice (Fig. 1b and Supplementary Figure 1b). The frequency and absolute numbers of CD4+ T-cells were also significantly reduced in the lungs of Bcl11bfl/fl dLck-iCre mice induced with HDM-asthma (Fig. 1c, d). The frequency of CD4+ T-cells within the mediastinal lymph nodes (mediLNs) was also reduced in Bcl11bfl/fl dLck-iCre mice, however not as severely as in the lung (Supplementary Figure 1c). Taken together, these results demonstrate that the removal of Bcl11b from mature T-cells results in reduced pulmonary pathology after induction of allergic asthma, which was associated with reduced lung eosinophils and CD4+ T-cells.
Bcl11bfl/fl dLck-iCre mice have reduced pulmonary inflammation during allergic asthma. a H&E staining of the lung parenchyma sections from asthmatic (n = 4) and naive mice (n = 2) 29 days after the initial HDM sensitization. Data are representative of two independent experiments. b Absolute number (left panel) and frequencies (right panel) of eosinophils in the lung parenchyma of HDM-asthma-induced Bcl11bfl/fl (n = 8) mice and Bcl11bfl/fl dLck-iCre (n = 6) mice. Eosinophils were identified by gating, presented in Supplementary Figure 1b. Data are representative of three independent experiments. c Flow cytometry analysis of CD4+ and CD8+ T-cell populations in the lung parenchyma of the indicated groups of HDM-asthma-induced mice. d Absolute number (left panel) and frequencies (right panel) of CD4+ T-cells in the lung parenchyma of HDM-asthma-induced Bcl11bfl/fl (n = 8) and Bcl11bfl/fl dLck-iCre (n = 6) mice. Data are representative of three independent experiments. All statistical values in this figure were calculated using Student’s t-test. All error bars shown in this figure are of standard deviation
Absence of Bcl11b in T-cells reduces anti-helminth response
Given the chronic nature of allergic asthma, we investigated the immune response to infection with intestinal helminth H. polygyrus bakeri, known to induce a strong primary Th2 response. Unlike many parasitic nematodes that migrate to other mucosal sites, the entire life cycle of H. polygyrus bakeri occurs within the gastrointestinal tract35. We found that on day 10 post inoculation (p.i.) (Supplementary Figure 2a), the Bcl11bfl/fl dLck-iCre mice had a significant increase in fecal egg burden, compared to WT mice (Fig. 2a). The Flow cytometry analysis showed a reduction in the absolute numbers of CD4+ T-cells in the mesenteric lymph nodes (mLNs) and Peyer’s patches (PPs) of Bcl11bfl/fl dLck-iCre mice (Fig. 2b, c), however the numbers were increased in the small intestine lamina propria (SILP), as were their relative frequencies (Fig. 2d and Supplementary Figure 2f-g). This increase in SILP CD4+ T-cells was found to be related to the upregulation of CCR9 on Bcl11b-deficient CD4+ T-cells (Supplementary Figure 2h). Thus, despite the increased abundance of CD4+ T-cells in the SILP, the fecal egg burden was higher in Bcl11bfl/fl dLck-iCre mice compared to WT mice, indicating a less robust Th2 response.
Bcl11bfl/fl dLck-iCre mice have elevated helminth egg burden in feces following infection with Heligmosomoides polygyrus bakeri. a Total egg burden in feces of infected Bcl11bfl/fl dLck-iCre mice (n = 16) and Bcl11bfl/fl control mice (n = 16). Data are representative of nine independent experiments. b Absolute numbers of total leukocytes (n = 8) and CD4+ T-cells (n = 4) from the mesenteric (m)LNs of helminth-infected mice. c Absolute numbers of total leukocytes (Bcl11bfl/fl: n = 10; Bcl11bfl/fl dLck-iCre: n = 9) and CD4+ T-cells (Bcl11bfl/fl: n = 8; Bcl11bfl/fl dLck-iCre: n = 6) from the Peyer’s patches (PPs) of helminth-infected mice from the indicated groups. d Absolute numbers of total leukocytes and CD4+ T-cells from the small intestine lamina propria (SILP) of helminth-infected Bcl11bfl/fl (n = 4) and Bcl11bfl/fl dLck-iCre (n = 3) mice. Data are representative of three independent experiments. All statistical values in this figure were calculated using Student’s t-test. All error bars shown in this figure are of standard deviation
Bcl11b-deficient T-helper cells downregulate Th2 mRNAs
To establish the mechanisms associated with reduced Th2 response in asthmatic and helminth-infected Bcl11bfl/fl dLck-iCre mice, we next performed RNA-sequencing on CD4+ T-cells isolated from: (1) mediLNs of asthma-induced Bcl11bfl/fl dLck-iCre and WT mice, and (2) mLNs of helminth-infected mice, and found that a large number of mRNAs significantly changed (Supplementary Figure 3). In regard to the Th2 program, we found that the levels of mRNAs for Gata3 and the Th2 cytokines Il4, Il5, and Il13 were all significantly reduced in the CD4+ T-cells from Bcl11bfl/fl dLck-iCre mice (Fig. 3a). We also detected an increase in several Th17 mRNAs, namely for Rorc and Ccr6, with Il17a, and Il23r showing a similar trend (Fig. 3b). Given that Bcl11bfl/fl dLck-iCre mice show a less robust Th2 response, we further enriched the IL-4-secreting CD4+ T-cell population and even in these conditions, the levels of Il4, Il13, and Gata3 mRNAs were still significantly reduced in the absence of Bcl11b, with a similar trend in Il5 mRNA (Fig. 3d). In addition, the previously observed increases in Th17 mRNAs, including for Rorc, Ccr6, Il17a, and Il23r, were amplified in the Bcl11b-deficient IL-4+ CD4+ T-cell population (Fig. 3e). While the levels of Ifng transcripts in the total CD4+ T-cell population were approximately equal, there was an increase in Ifng mRNA levels in the IL-4-enriched CD4+ T-cell population of the Bcl11bfl/fl dLck-iCre mice, compared to WT mice (Fig. 3c, f). Tbx21 mRNA encoding the Th1 signature transcription factor T-bet was upregulated as well, both in the IL-4-enriched CD4+ T-cell population and total CD4+ T-cells (Fig. 3c, f). Finally, we found no change in Gfi1 mRNA (Fig. 3g), known to be downregulated in Bcl11b-deficient ILC2s33, or Zbtb7b (Fig. 3g), previously found upregulated in Bcl11b-deficient DP thymocytes36.
Bcl11b-deficient CD4+ T-cells downregulate the Th2 program and upregulate Th17 and Th1 genes. a–c Box and whisker plots of normalized counts for specific Th2-associated (a), Th17-associated (b), and Th1-associated (c) mRNAs derived from RNA-Seq on total CD4+ T-cells from the mLNs of H. polygyrus bakeri infected Bcl11bfl/fl dLck-iCre mice (n = 4) and Bcl11bfl/fl control mice (n = 4). Normalized counts and statistical analyses were taken from DESeq2 analysis software. Data are derived from two independent experiments. p values have been adjusted for the FDR (false discovery rate) by the Benjamini–Hochberg adjustment. d–f Box and whisker plots of normalized counts for specific Th2-associated (d), Th17-associated (e), and Th1-associated (f) mRNAs derived from RNA-Seq on IL-4+-enriched CD4+ T-cells from the lung parenchyma of asthma-induced Bcl11bfl/fl dLck-iCre mice (n = 4) and Bcl11bfl/fl control mice (n = 4). g Box and whisker plots of normalized counts for Gfi1 and Zbtb7b mRNA from RNA-Seq on IL-4+-enriched CD4+ T-cells from the lung parenchyma of asthma-induced Bcl11bfl/fl dLck-iCre mice (n = 4) and Bcl11bfl/fl control mice (n = 4). Normalized counts and statistical analyses were taken from DESeq2 analysis software. p values have been adjusted for the FDR (false discovery rate) by the Benjamini–Hochberg adjustment. All error bars shown are of standard deviation
These results, taken together, suggest that, in the context of Th2-mediated diseases, Bcl11b is necessary for Th2 cytokine and Gata3 gene expression, as well as for the repression of critical Th17 and Th1 cell genes.
Reduction in Th2 program is caused by intrinsic defects
We next evaluated the protein levels of GATA3 and Th2 cytokines in the T-helper cells of mice induced with HDM-asthma or infected with helminths. We found that the frequencies of GATA3+IL-4+, GATA3+IL-5+, and GATA3+IL-13+ CD4+ T-cells in the lungs of asthma-induced Bcl11bfl/fl dLck-iCre mice were reduced, as were the frequencies of GATA3+, IL-4+, IL5+, and IL-13+ CD4+ T-cells. (Fig. 4a, b). When evaluating H. polygyrus bakeri-infected mice, we noticed a similar reduction in Th2 cytokines and GATA3 within the CD4+ T-cells from the mLNs (Fig. 4c, d) and SILP (Supplementary Figure 4a-c). To further determine the ability of Bcl11b-deficient T-helper cells to differentiate in vivo, we separated the GATA3+ CD4+ T-cells from the mLNs of helminth-infected mice into GATA3hi and GATA3lo populations, and also gated separately on Bcl11b− and Bcl11b+ CD4+ T-cells of Bcl11bfl/fl dLck-iCre mice (Fig. 4e, f). The GATA3hi/GATA3lo ratio was 2.6 within the CD4+ T-cell population of WT mice (Fig. 4e, f), while this ratio was only 0.52 in the Bcl11b-deficient CD4+ T-cell population of Bcl11bfl/fl dLck-iCre mice (Fig. 4e, f). Even the small population of Bcl11b+ “escaper” CD4+ T-cells from Bcl11bfl/fl dLck-iCre mice had a similarly reduced ratio of GATA3hi/GATA3lo cells (Fig. 4e, f), likely due to the overall reduction in IL-4. Within the GATA3hi population of WT mice, the frequency of IL4+ cells was around 80%. This frequency was reduced by nearly half in both the Bcl11b− and Bcl11b+ GATA3hi cells of Bcl11bfl/fl dLck-iCre mice (Fig. 4e, f). The frequency of IL-4+ cells within the GATA3lo cells was only one-third of the GATA3hi cells in the WT mice, and was further reduced in both the Bcl11b− and Bcl11b+ cells of Bcl11bfl/fl dLck-iCre mice (Fig. 4e, f). These results suggest that (1) Bcl11b-deficient CD4+ T-cells are unable to completely differentiate into the Th2 lineage, and (2) even those cells that reach high levels of GATA3 cannot produce normal levels of IL-4 in the absence of Bcl11b.
Frequencies of CD4+ T-cells expressing Gata3 and Th2 cytokines in asthma and helminth infection are reduced in Bcl11bfl/fl dLck-iCre mice by an intrinsic defect. a Flow cytometry analysis of IL-4 (left column), IL-5 (middle column), and IL-13 (right column) against GATA3 in the CD4+ T-cell population of the lung parenchyma of HDM asthma-induced mice. b Frequencies of IL-4+ (n = 4), IL-5+ (n = 4), and IL-13+ (n = 8) (top panel) and GATA3+ (n = 10) (bottom panel) CD4+ T-cells from the lungs of the indicated groups. Data are derived from two (IL-4, IL-5, and IL-13 data) or five (GATA3 data) independent experiments. c Flow cytometry analysis of IL-4 (left column), IL-5 (middle column), and IL-13 (right column) against GATA3 in the CD4+ T-cell population from the mesenteric (m)LNs of H. polygyrus infected mice. d Frequencies of IL-4+ (n = 4), IL-5+ (n = 4), and IL-13+ (n = 4) (top panel) and GATA3+ (n = 8) (bottom panel) CD4+ T-cells from mLNs of the indicated groups. Data are derived from two (IL-4, IL-5, and IL-13) or four (GATA3) independent experiments. e Flow cytometry analysis of GATA3 in CD4+ T-cells (left column) and IL-4 (middle and right columns) in the GATA3hi (middle column) and GATA3lo (right column) CD4+ T-cells from the mLNs of H. polygyrus bakeri infected mice. f The ratio of GATA3hi/GATA3lo CD4+ T-cells (top) and frequencies of IL-4 secreting cells within the GATA3hi and GATA3lo CD4+ T-cells (bottom) from the mLNs of H. polygyrus bakeri infected WT (n = 4) and Bcl11bfl/fl dLck-iCre (n = 3) mice. Data shown are from two independent experiments. g Flow cytometry analysis of IL-4 and GATA3 in CD4+ T-cells from the mLNs of mixed bone-marrow chimeric mice infected with H. polygyrus bakeri. Cells from WT donors are CD45.1/CD45.2, while cells from Bcl11bfl/fl dLck-iCre donors are CD45.2. h Frequencies of IL-4+GATA3+ cells from the mLNs of mixed bone-marrow chimeric mice (n = 6) infected with H. polygyrus bakeri. Data shown are from two independent experiments. All statistical values in this figure were calculated using Student’s t-test. All error bars shown in this figure are of standard deviation
Importantly, the upregulation of the Rorc and Tbx21 mRNAs was not reflected at protein level (Supplementary Figure 5a-d), making it unlikely to contribute to the phenotype. In addition, proliferation of Bcl11b-deficient T-helper cells was not affected, as demonstrated by the similar Ki-67+ population within CD4+ T-cells from Bcl11bfl/fl dLck-iCre and WT mice (Supplementary Figure 5c–d).
To establish whether the reduction in Gata3 and IL-4 levels in Bcl11b− CD4+ T-cells is cell intrinsic, we generated mixed bone-marrow chimeras. Lethally irradiated recipient CD45.1 mice were reconstituted with cells from Bcl11bfl/fl (CD45.1/2) and Bcl11bfl/fl dLck-iCre (CD45.2) mice at a 1:1 ratio. Following reconstitution, the recipient mice were infected with H. polygyrus bakeri. Our results show that even in conditions in which Bcl11b+ cells are present in equal numbers to Bcl11b− cells, GATA3 and IL-4 levels remained reduced in the Bcl11b− cells (Fig. 4g, h), suggesting that these alterations are intrinsic.
These results demonstrate that in the absence of Bcl11b, CD4+ T-cells have an altered ability to differentiate into Th2 lineage properly in vivo, with diminished GATA3 and Th2 cytokine levels, caused by intrinsic defects.
Bcl11b binding at Th2 cytokine locus and Gata3
To further determine if Bcl11b directly mediates the observed changes in the Th2 program, we performed Bcl11b ChIP-seq on CD4+ T-cells from the mLNs of H. polygyrus bakeri infected mice. Using the MEME-ChIP motif analysis software37, we established the conserved binding motifs for Bcl11b, of which the top two are shown (Fig. 5a). The first identified motif is a known Ets-1 binding motif, while the second motif, identical to a previously published Bcl11b binding site in striatal neuronal cells38, is a Runx binding motif39. Bcl11b binding sites were found to have increased presence at regions greater than 5 kb from the TSS (Fig. 5b), suggesting that, overall, Bcl11b may normally act through distal regulatory elements, such as enhancers and silencers, rather than through promoters.
Bcl11b binds at the Gata3 locus and downstream and at the Il4 HS IV silencer but not at the Th2-LCR or Th2-cytokine promoters in CD4+ T-cells. a The top two Bcl11b binding motifs discovered using MEME-ChIP motif analysis software from Bcl11b ChIP-seq of CD4+ T-cells from WT mice infected (n = 2) with H. polygyrus bakeri. b Density distribution of Bcl11b binding relative to the nearest transcription start site (TSS). Data obtained from Genomic Regions Enrichment of Annotations Tool (GREAT). c–e Integrative Genomics Viewer visualizations of Bcl11b ChIP-Seq tracks at the Th2 cytokine locus (c, Il4 HS IV silencer is highlighted), Gata3 (d intronic regions are highlighted), and (e conserved non-coding binding sites downstream of Gata3 are highlighted) from naive (n = 2) (c) or helminth-infected (n = 2) (c–e) mice. ChIP-Seq track scale bar normalized to sequences per million reads (SPMR)
Among the Th2 genes, the most striking finding was the fact that Bcl11b did not bind to Il4, Il13, or Il5 promoters or to the Th2 LCR located within Rad50 gene (Fig. 5c). However, Bcl11b bound to the Il4 HS IV silencer, located downstream of the 3′ end of the Il4 gene (Fig. 5c), suggesting that Bcl11b may be necessary to limit the accessibility of Il4 HS IV silencer in Th2 cells. In addition, we found Bcl11b binding to conserved intronic sites within the Gata3 locus (Fig. 5c), as well as to other conserved non-coding regions downstream of the Gata3 locus (Fig. 5e), which suggests that Bcl11b is potentially involved in direct regulation of Gata3 gene expression.
Bcl11b regulates chromatin opening at Th2 cytokine locus
We next performed the Assay for Transposase-Accessible Chromatin and sequencing (ATAC-seq) to evaluate the changes in chromatin accessibility40 in CD4+ T-cells isolated from the lung of Bcl11bfl/fl dLck-iCre and WT mice with HDM-induced asthma (Fig. 6). We identified over 60,000 accessible regions in CD4+ T-cells from either WT or Bcl11bfl/fl dLck-iCre mice. Of these, 9516 were differentially accessible between the two groups (Fig. 6a). The majority of differentially accessible regions (~65%) were unique to Bcl11b-deficient cells, while relatively few (~8%) were unique to WT cells (Fig. 6b). This profile suggests that in these cells, Bcl11b plays a larger role in restricting chromatin accessibility than in opening it. Furthermore, using Genomic Regions Enrichment of Annotations Tool (GREAT)41, we found that the differentially accessible regions unique to one group were more likely to be found further away from the TSSs, while those regions common to both groups had a higher probability of being found closer to the TSSs (Fig. 6c). This suggests that Bcl11b is more likely to regulate enhancers and silencers rather than promoters, similarly to what we found for its binding relative to the TSSs (Fig. 5b).
Removal of Bcl11b results in reduced chromatin accessibility at key Th2 loci and increased accessibility at the Il4 HS IV silencer. a Scatterplot of normalized ATAC-Seq coverage per peak. b Venn diagram of the distribution of significantly differentially accessible regions. Significance was calculated by MAnorm analysis and was defined as |log2(fold-change)| > 1 and p < 0.05. p value was adjusted for the FDR (false discovery rate) by the Benjamini–Hochberg adjustment. c Density distribution of accessible regions relative to the nearest transcription start site (TSS). Data obtained from Genomic Regions Enrichment of Annotations Tool (GREAT). d–g Integrative Genomics Viewer visualizations of ATAC-Seq tracks at Rad50 Th2 LCR (RHS V highlighted) (d), Il5 (promoter highlighted) (e), Il13 (promoter highlighted) (f), and Il4 (Il4 HS IV silencer highlighted) (g). ATAC-Seq track scale bar normalized to SPMR
We focused on genes related to Th2 response, and found that the Rad50 HS 5 (RHS V) within the Th2 cytokine LCR had significantly reduced accessibility in Bcl11b-deficient T-helper cells compared to WT cells from asthma-induced mice (Fig. 6d), despite the fact that Bcl11b did not bind to this region (Fig. 5c). In addition, the accessibility at Il5 and Il13 promoters was reduced in the absence of Bcl11b (Fig. 6e, f), again without Bcl11b binding at these promoters (Fig. 5c). However, there was a significant increase in the chromatin accessibility at the Il4 HS IV silencer both in Th2 cells and in naive CD4+ T-cells (Fig. 6g), where Bcl11b was found to bind (Fig. 5c).
These results show that the absence of Bcl11b in T-helper cells in Th2 conditions in vivo causes a failure in chromatin opening at the Th2 LCR, Il5 and Il13 promoters, and an inability to reduce the chromatin accessibility at the Il4 HS IV silencer, including in naive cells.
Other genes regulated by Bcl11b in Th2 cells
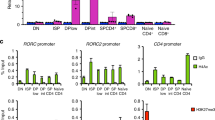
As shown above mRNAs for Th17 and Th1 genes were upregulated in the absence of Bcl11b. While there was no binding of Bcl11b at Rorc, Tbx21, IL23R, and CCR6 loci, Bcl11b did bind to conserved non-coding regions adjacent to Ifng and Il17 loci (Supplementary Figure 6a-b), suggesting a direct mechanism of repression of their expression. In addition, absence of Bcl11b caused an increase in mRNAs for several myeloid genes, including Itgam (Cd11b), Itgax (Cd11c), Fcer1g and Fcgr3 (Supplementary Figure 6c), and of mRNAs encoding killer cell lectin-like receptors, including Klrc1 and Klrd1 (Supplementary Figure 6d). Bcl11b directly associated with non-coding regions in close proximity to these genes (Supplementary Figure 6e), as well as within non-coding regions in the Spi1 gene, encoding PU.1 transcription factor, which, was however not upregulated at the mRNA level (Supplementary Figure 6f). These results suggest that Bcl11b directly restricts expression of Ifng and Il17 and of several myeloid and NK genes in Th2 cells.
Bcl11b-deficient T-helper cells have elevated Runx3
As shown above, absence of Bcl11b caused increased chromatin accessibility at the Il4 HS IV silencer. In Th1 cells, Runx3 and T-bet synergistically bind to Il4 HS IV silencer12 to repress Il4 transcription, however Runx3 alone is sufficient to repress Il4 in Th1 cells11,12, even though both Runx3 and T-bet are needed to promote Ifng expression. Both Runx3 and Tbx21 mRNAs were significantly increased in the absence of Bcl11b (Figs. 3c, f and 7a). However, while T-bet protein remained unchanged (Supplementary Figure 5a), Runx3 protein was considerably upregulated in CD4+ T-cells from both helminth-infected and naive Bcl11bfl/fl dLck-iCre mice (Fig. 7b–d). However Runx3 expression was not upregulated in CD4 SP thymocytes of Bcl11bfl/fl dLck-iCre mice, and was expressed at equivalent levels to WT mice in CD8 SP thymocytes (Supplementary Figure 7), demonstrating that the phenotypes observed in the periphery are unlikely to be developmental in origin.
Bcl11b binds to enhancers upstream of Runx3 locus to restrain Runx3 expression in CD4+ T-cells. a Box and whisker plots of normalized counts for Runx3 from RNA-Seq on CD4+ T-cells from the mesenteric (m)LNs of helminth-infected mice. Data (n = 4) are derived from two independent experiments. p values have been adjusted for the FDR (false discovery rate) by the Benjamini–Hochberg adjustment. b Flow cytometry analysis of Runx3 in CD4+ T-cells from the mLNs of H. polygyrus bakeri infected Bcl11bfl/fl (n = 10) and Bcl11bfl/fl dLck-iCre (n = 12) mice (top) and their relative frequencies (bottom). Data shown are from five independent experiments. c Flow cytometry analysis of Runx3 in CD4+ T-cells from the mLNs of naive Bcl11bfl/fl (n = 4) and Bcl11bfl/fl dLck-iCre (n = 3) mice (top) and their relative frequencies (bottom). Data shown are from two independent experiments. d Flow cytometry analysis of Runx3 vs. Gata3 in total CD4+ T-cells from the mLNs of helminth-infected mice. Data representative of three independent experiments. e Runx3 enrichment at the HS IV silencer determined by Runx3 ChIP-qPCR performed on CD4+ T-cells isolated from the mLNs of helminth-infected mice or the spleen and peripheral lymph nodes of naive mice. ChIP-PCR enrichment is expressed as a percentage of input (n = 2). f Integrative Genomics Viewer visualizations of Bcl11b ChIP-Seq in total thymocytes (deposited data from Kojo et al.43) (top track), Bcl11b ChIP-Seq in CD8-enriched thymocytes from naive mice (second track), Bcl11b ChIP-Seq in naive in CD4+ T-cells (tracks 3–4), Bcl11b ChIP-Seq in CD4+ T-cells from H. polygyrus infected mice (tracks 5–6) and H3K27ac ChIP-Seq (tracks 7–8) in WT and Bcl11b-deficient CD4+ T-cells from H. polygyrus infected mice at Runx3 locus. g Histogram of Runx3 in CD4+ T-cells from the mLNs of mixed bone-marrow chimeric mice infected with H. polygyrus bakeri. Gating strategy (shown in Fig. 4g) was first on CD4+ T-cells, and then on CD45.1+CD45.2+ (WT) (filled histogram) and CD45.2+ (Bcl11b−) (dashed, open histogram). h Frequencies of Runx3+ cells in the CD4+ T-cells from the mLNs of mixed bone-marrow chimeric mice (n = 5) infected with H. polygyrus bakeri. Data shown are from two independent experiments. All statistical values in this figure were calculated using Student’s t-test except for panel a, which was calculated using DESeq2 analysis software. All error bars shown in this figure are of standard deviation
Given that Runx3 is known to associate with the Il4 HS IV silencer to block Il4 transcription in Th1 cells12, we investigated whether the increased Runx3 in the absence of Bcl11b resulted in enhanced Runx3 binding to the Il4 HS IV silencer by performing Runx3 ChIP-qPCR. We indeed found elevated Runx3 binding to the more accessible Il4 HS IV silencer in CD4+ T-cells from naive and helminth-infected Bcl11bfl/fl dLck-iCre mice compared to WT (Fig. 7e). Together, these results show that in the absence of Bcl11b, Runx3 levels are elevated, and the available Runx3 binds to the more accessible Il4 HS IV silencer both in CD4+ T-cells of helminth-infected and naive mice.
Bcl11b directly binds to and represses Runx3 gene expression
Given the elevated Runx3 levels, we next examined the Bcl11b ChIP-seq data and found enrichment of Bcl11b binding to two conserved non-coding regions, namely −39 kb and −18 kb upstream of the Runx3 TSS in CD4+ T-cells from naive and helminth-infected mice (Fig. 7f). To further evaluate the chromatin activity at these sites, we performed ChIP-seq for H3K27ac, a histone modification known to be associated with active enhancers and promoters of actively transcribed genes42. Absence of Bcl11b caused increased H3K27ac at both the −39 kb and −18 kb regions (Fig. 7f), suggesting that Bcl11b directly restricts expression of Runx3 by binding to and deactivating upstream Runx3 conserved enhancers in CD4+ T-cells. Further supporting our results is a recent publication in which deletion of the −39 kb cis-regulatory region bound by Bcl11b diminished the activity of a Runx3-reporter in CD8 SP thymocytes43. We also performed Bcl11b ChIP-seq in CD8-enriched thymocytes (mainly DP and CD8 SP thymocytes) and found Bcl11b binding at the −39 kb, −21 kb, and −18 kb cis-regulatory conserved sites, as well as at the P1 promoter (Fig. 7f), critical for expression of Runx3 in CD8+ T-cells44. Thus, in thymocytes, Bcl11b binds to the cis-regulatory regions located at −39 kb, −21 kb, and −18 kb and to the P1 promoter (Fig. 7f), while in naive and Th2 CD4+ T-cells Bcl11b binds only to the −39 kb and −18 kb conserved sites (Fig. 7f).
Finally, we evaluated Runx3 in CD4+ T-cells of mixed bone-marrow chimeric mice infected with H. polygyrus bakeri. We found that Runx3 was upregulated only in the CD4+ T-cells which had Bcl11b removed and not in WT cells (Fig. 7g, h), demonstrating that the upregulation of Runx3 in the absence of Bcl11b is intrinsic.
Thus these results demonstrate that Bcl11b directly restricts expression of Runx3 in Th2 cells and in naive CD4+ T-cells by binding to and deactivating upstream Runx3 enhancers.
Discussion
In this study, we demonstrate that Bcl11b plays a critical role in the differentiation of Th2 cells in vivo. Bcl11b has been previously shown to repress GATA3 and IL-4 in Th17 cells during EAE30. In addition, Bcl11b−/− CD4+ T-cells were shown to differentiate normally into Th2 cells in vitro, with their polarization being even slightly enhanced, compared to WT CD4+ T-cells30. However, in ILC2s, which are functionally similar to Th2 cells, Bcl11b was needed to sustain key type 2 transcription factors and cytokines and to block the ILC3 program33. We report here that Bcl11b is a critical transcription factor for licensing Th2 differentiation in vivo. Specifically, in response to allergen-induced asthma and intestinal helminth infection, CD4+ T-cells of Bcl11bfl/fl dLck-iCre mice failed to efficiently differentiate into Th2 cells, despite the fact that they expanded normally. Consequently, Bcl11bfl/fl dLck-iCre mice had reduced pulmonary pathology associated with the induction of allergic asthma, while having increased fecal egg burden following helminth infection. Transcriptome, chromatin accessibility and ChIP-seq analyses revealed the mechanisms by which Bcl11b regulated Th2 cell differentiation in vivo. In addition to the Th2 cytokines, GATA3 expression was reduced both at protein and mRNA levels. GATA3 has been shown to be indispensable for the initiation and propagation of Th2 differentiation45. The reduction in GATA3 was similar to the defects we previously found in the absence Bcl11b in ILC2s33, where, in addition, Gfi1 and Rora were also downregulated. The reduction in GATA3 in ILC2s was shown to be dependent on diminished Gfi1 expression. However, we did not find any changes in Gfi1 expression in Bcl11b-deficient Th2 cells, suggesting that the control of Gfi1 by Bcl11b is cell-type dependent. We did find binding of Bcl11b to several conserved intronic regions of Gata3 and non-coding sequences downstream of Gata3 locus. Whether this binding has functional consequences, or if Bcl11b binds to these sites in ILC2s, remains to be established. The reduced IL-4 level in the absence of Bcl11b may also contribute to diminished Gata3 expression, and it remains to be established what transcription factors mediate this regulation.
GATA3 itself is known to act at the Th2 LCR and at the Il5 and Il13 gene promoters to enhance their transcription46. The Th2 LCR RHS V enhances Il4 transcription through interactions with the Il4 promoter, with GATA3 playing a central role in this process10. Bcl11b-ChIP-seq analysis showed no binding of Bcl11b at Th2-LCR, or Th2-cytokine promoters in Th2 cells; however, ATAC-seq showed reduced chromatin accessibility at the Th2 LCR RHS V and at the Il5 and Il13 promoters in the absence of Bcl11b. Thus the reduction in accessibility can be related, at least in part, to the decreased GATA3 levels in the absence of Bcl11b. In addition to the Th2 LCR and Il4 promoter, Il4 expression is also regulated through the Il4 HS IV silencer, which functions to repress Il4 expression in Th1 cells in vivo11,12. A second defect of Bcl11b− T-helper cells during Th2 response was the high chromatin accessibility at the Il4 HS IV silencer, much more than in WT Th2 cells, similar to Th1 cells. This increase was also seen in the naive state. We found that Bcl11b directly binds to Il4 HS IV silencer in naive and CD4+ T-cells of mice infected with helminths, demonstrating that Bcl11b plays a critical role in directly limiting Il4 HS IV silencer accessibility in Th2 and naive CD4+ T-cells. Previous studies have demonstrated that Runx3, normally expressed in Th1 cells and CD8+ T-cells, binds to the Il4 HS IV silencer in Th1 cells and represses Il4 expression12. In relation to this, Bcl11b− T-helper cells from naive mice as well as from mice infected with helminths, had a third defect: showing a major elevation in Runx3 levels compared to Bcl11b-sufficient cells. In addition, Runx3 showed increased association with the Il4 HS IV silencer in both Bcl11b− CD4+ T-cells from naive and helminth-infected mice. Interestingly, the second motif that we identified as being bound by Bcl11b, “ACCACA”, appears to be a Runx binding motif39. Thus, absence of Bcl11b results in availability and increased accessibility of Il4 HS IV silencer and elevated Runx3, which now can bind at the silencer. This, together with reduced levels of GATA3 cause diminished Il4 expression and reduced ability to efficiently differentiate into Th2 lineage in vivo.
Runx3 has also been shown to be important in ILC1 and ILC3 development, but not in ILC2 development44. In ILC3s, Runx3 positively regulates Rorc expression, which in turn increases the expression of Ahr44. It remains to be established whether in Bcl11b-deficient ILC2s, Runx3 is derepressed in a manner similar to Bcl11b− T-helper cells, which then may also contribute to the shift from ILC2 to ILC3 program33, a possibility which also exists in iNKT cells, where the absence of Bcl11b caused derepression of iNKT17 genes and reduction of the other iNKT cell subset genes26.
The disparities between the actions of Bcl11b on Th2 differentiation in vivo and in vitro have been of particular interest to us. We speculate that the constant supply of exogenous IL-4 during in vitro polarization is key to the observed changes, as previous reports have shown that certain defects in Th2 differentiation, such as those observed related to Notch signaling, can be compensated for in this way4,47. It is possible that by providing constant, high amounts of exogenous IL-4 during the in vitro polarization, a Bcl11b-deficient cell is able to gradually overcome its defect in IL-4 production and begins upregulating GATA3 to a level sufficient to compensate the defect, and the excess in Runx3 is eventually overcame, allowing for the stabilization of the Th2 lineage in vitro.
We found that Bcl11b binds to several conserved cis-regulatory regions upstream of Runx3, namely at −39 kb, −21 kb, and −18 kb, as well as to the P1 promoter in CD8-enriched thymocytes, while in naive and Th2 CD4+ T-cells, it only bound to the −39 kb and −18 kb sites. Our finding that removal of Bcl11b results in enrichment of H3K27ac at the −39 kb and −18kb sites suggests that Bcl11b inactivates these enhancers in Th2 cells and likely in naive CD4+ T-cells. Very recently, in addition to our findings, Bcl11b was found to bind to the Runx3 cis-regulatory regions −39 kb and −21 kb sites in total thymocytes43. When these regions were deleted, a reduction in Runx3-reporter expression was observed in CD8 SP thymocytes, indicating that these regions act as enhancers for Runx3 in CD8 SP thymocytes43, and supporting the idea that in naive and Th2 cells, Bcl11b inactivates Runx3 enhancers, thus blocking its expression. It remains to be established whether Bcl11b binds to these Runx3 cis-regulatory regions in DP, CD4 SP thymocytes, and/or CD8 SP thymocytes, given that the ChIP-seq analysis was conducted in total thymocytes43, and in CD8-enriched thymocytes (this study). Interestingly, Thpok was also found to bind to the −39 kb and −21 kb sites in total thymocytes43. We did not find any changes in Thpok mRNA levels in the absence of Bcl11b in Th2 cells. Thus Runx3 repression in mature naive and Th2 CD4+ T-cells requires Bcl11b, and Thpok alone is likely not sufficient to exert this function. However, Thpok is essential in blocking Runx-dependent differentiation toward CD8 lineage48. We did not notice any increase in CD8+ T-cells versus CD4+ T-cells as a consequence of Runx3 upregulation, likely due to the fact that removal of Bcl11b occurred after commitment to CD4 SP and CD8 SP thymocytes. In addition, CD8 expression was not detected in CD4+ T-cells. It remains to be established how Bcl11b regulates Runx3 in Th1 cells and CD8+ T-cells, where Runx3 is expressed49.
Similar to ILC2s, in Th2 cells, Bcl11b is also required to repress type 3 program including Rorc and Il17, however different from ILC2s, the proteins could not be detected in Bcl11b− Th2 cells, thus unlikely to participate in the phenotype. Tbx21 and Ifng mRNAs, but not proteins, were also upregulated. Except for Il17, Ifng, and Runx3, Bcl11b did not bind any other Th17 or Th1 loci or adjacent non-coding regions.
Bcl11b also repressed expression of several myeloid genes, including those encoding Cd11b, Cd11c, and Fc receptors, as well as of some killer cell lectin-like receptors and also bound to cis-regulatory regions within or upstream of these genes, suggesting that Bcl11b may directly repress their expression in differentiating Th2 cells.
In conclusion, we established here that the absence of Bcl11b in naive and during Th2 response in vivo results in derepression of Runx3, as well as provision of access for Runx3 to the Il4 HS IV silencer. These events, together with reduced GATA3, caused reduced chromatin accessibility at Th2 LCR, and Th2 gene promoters, further resulting in reduced Th2 differentiation. These results together suggest that Bcl11b primes naive CD4+ T-cells in such a way to be permissive for Th2 differentiation, and absence of Bcl11b causes a failure in efficient licensing of Th2 lineage.
Methods
Mice
Bcl11bfl/fl dLck-iCre mice on a C57BL/6 were previously described28. All mice were raised and housed in pathogen-free conditions in the University of Florida Animal Facilities except mice infected with H. polygyrus, which were transferred to a BSL2 facility prior to infection. All experiments were conducted on 8–12-week-old male and female mice. All mouse work was approved by the University of Florida Institutional Animal Care and Use Committee. No statistical methods were used to predetermine sample size.
Induction of allergic asthma by HDM extract
8–12-week-old mice were sensitized to house-dust-mite antigen by seven intraperitoneal injections of 100 μL PBS containing 50 µg HDM extract (D. pteronyssinus, Greer Labs) on days 1, 3, 5, 7, 9, 11, and 13. Then, on days 25, 26, and 27, mice were challenged intranasally (i.n.) with 20 μL PBS containing 50 µg HDM extract. Mice were anesthetized with isoflurane prior to each i.n. challenge. Mice were sacrificed on day 29.
Lung digestion for isolation of leukocytes
Lungs were initially perfused through the right ventricle with 10 mL cold PBS. The lungs were then excised and placed in a C tube (Miltenyi Biotech) containing 5 mL cold PBS and subsequently minced on a gentleMACS Dissociator (Miltenyi Biotech). The lungs were then digested for 45 min at 37 °C with the addition of 0.2 Wünsch units/mL of Liberase TM (Roche) and 0.15 mg/mL DNase I (Roche). Digested tissue was filtered through a 70-μm strainer and washed with complete RPMI. Epithelial cells were removed by density separation using a 30%/70% discontinuous Percoll gradient.
Helminth infection
8–12-week-old mice were infected with H. polygyrus via oral gavage with 200 μL water containing 200 L3 larvae. Fecal pellets were collected immediately before euthanasia on day 12 post infection, with the eggs/g feces serving as a readout of overall worm fitness.
Isolation of leukocytes from the SILP
Small intestinal lamina propria preparation methodology was adapted from previous study50. Small intestines were flushed with cold PBS and PPs were removed and set aside. Tissue was cleaned and washed once with 5 mM DTT and thrice with 5 mM EDTA. SILP was then digested at 37 °C for 30 min with 0.2 Wünsch units/mL of Liberase TM (Roche) and 0.1 mg/mL DNase I (Roche). Digested tissue was filtered through a 70-μm strainer and washed with complete RPMI. Remaining epithelial cells were removed by density separation using a 30%/70% discontinuous Percoll gradient.
Isolation of CD4+ T-cells from total leukocytes
For leukocytes prepared from lung tissue, CD4+ T-cells were purified by FACS, using a BioRad S3e Cell Sorter. For cells from the mesenteric and mediastinal lymph nodes, CD4+ T-cells were purified using Dynabeads Untouched Mouse CD4 Cell kit (Invitrogen).
Enrichment of IL-4+CD4+ T-cells from total leukocytes
Labeling and enrichment of IL-4+ cells from pre-negatively selected CD4+ T-cells was performed using the Mouse IL-4 Secretion Assay kit from Miltenyi Biotech (#130-00-515). Enrichment was verified to be 40%, by flow cytometry.
RNA-Seq
RNA was extracted from CD4+ T-cells purified from the mLNs of H. polygyrus infected mice or the mediLNs of asthmatic mice using RNeasy Plus Micro Kit (QIAGEN). cDNA libraries were prepared off-site and sequencing was performed on a NextSeq 500. Sequencing reads were processed and analyzed using a custom pipeline. Fastq files were initially trimmed using seqtk51. Quality of the fastq files were assessed via FastQC52. Trimmed reads were aligned to the mouse (mm10) genome using Hisat253. Htseq54 was utilized to obtain transcript counts for each sample, which were then run through DeSeq255 for differential analysis.
ATAC-Seq
DNA from 50,000 CD4+ T-cells was extracted and processed for ATAC-Seq as described by others40. Library quality and adapter dimer contamination was assessed on a Bioanalyzer (Agilent, #5067-4626). Libraries were run as PE75 on a NextSeq 500. Data analysis was performed using a custom pipeline based on a modified version of a previously published method56. Fastq files were initially trimmed using Trimmomatic57, then read quality was assessed using FastQC as before52. Reads were aligned to the mouse (mm10) genome using Bowtie258 and the resulting SAM file was pruned (Q > 30), converted into BAM format, and sorted using SAMtools59. Duplicate reads were identified and removed using Picard tools60. Peaks were called and bedgraph files were prepared using MACS2 peak calling software61. Bedgraph files were then visualized using Integrative Genomics Viewer (IGV)62.
Bcl11b ChIP-Seq
ChIP-Seq against Bcl11b was performed on CD4+ T-cells using the SimpleChIP Enzymatic Chromatin IP Kit from Cell Signaling Technologies (#9003), following their recommended protocol up to the library preparation with the following alterations: (1) 107 cells were used in place of 4 × 107; (2) formaldehyde fixation was performed in 1 mL; (3) Buffer A and Buffer B steps were all performed in 1 mL; (4) 2 μl of 1:10 diluted Micrococcal nuclease was determined to give the optimum digestion results by titration; (5) cocktail of two anti-Bcl11b antibodies were used (8 μg ab18465 and 3 μg of CST #12120 per ChIP); (6) final DNA purification was performed by phenol:chloroform:isoamyl alcohol extraction with MaXtract high density columns (Qiagen #129046). Libraries were prepared using NEBNext DNA Library Kit (NEB, #E7645), with quality being determined as above. Libraries were run as SE75 on a NextSeq 500. Fastq analysis performed as with ATAC-Seq, but with single-end rather than paired-end settings.
H3K27ac ChIP-Seq
ChIP-Seq against H3K27ac was performed on 100,000 CD4+ T-cells isolated from the lungs of HDM asthma-induced Bcl11bfl/fl dLck-iCre and Bcl11bfl/fl mice using the Low Cell ChIP-Seq kit from Active Motif (#104895), following their recommended protocol scaled to 100,000 cells. The antibody used was acetyl-histone H3 (Lys27) (D5E4) XP® Rabbit mAb from Cell Signaling Technologies (#8173) at the recommended 1:100 dilution. Libraries were run as SE75 on a NextSeq 500. Fastq analysis performed as with Bcl11b ChIP-Seq.
Mixed bone-marrow chimeras
To generate mixed bone-marrow chimeras, CD45.1 mice were irradiated twice with 500 rads, 4 h apart, on the same day prior to reconstitution with cells from CD45.1/CD45.2 Bcl11bfl/fl mice and CD45.2 Bcl11bfl/fl dLck-iCre mice at a 1:1 ratio. Recipient mice were then left for a minimum of 8 weeks (the first two of which were on antibiotics), at which point they were used for experimentation.
Cytokine stimulation for intracellular flow cytometry
For detection of cytokines by intracellular flow cytometry, cells were cultured at 37 °C and 5% CO2 for 4 h in IMDM media containing PMA (20 ng/ml) and Ionomycin (1 µg/ml). Brefeldin A (10 µg/ml) was added after 1 h. After incubation, cells were washed and stained with Fixable viability dye (Affymetrix) and surface markers. Cells were then fixed and permeabilized with Foxp3 Fix/Perm kit (Affymetrix, #00-5521), then subsequently stained for cytokines.
Flow cytometry
Flow cytometry was performed on an BD LSR II, with data acquired using BD FACS DIVA software. All data were analyzed using FlowJo (TreeStar).
Antibodies
The antibodies used either for staining for flow cytometry or for ChIP-seq and ChIP-PCR are shown in Table 1.
Statistical analyses
For non-sequencing experiments, differences between groups were calculated by a two-tailed Student’s t-test assuming unequal variance. For RNA-Seq experiments, significance was calculated by DeSeq2 with a false discover rate (FDR) cutoff of 0.05. For ATAC-Seq experiments, significant differences in enrichment between peaks was determined using an optimized MANorm script64,65 with the cutoff set at a minimum fold-change of 2 and significance of p < 0.05.
Data availability
RNA-seq, ChIP-seq, and ATAC-seq data shown in this study have been deposited in the Gene Expression Omnibus (GEO) database with accession GSE99599 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE99599).
All other relevant data and reagents are available from the authors.
Change history
19 July 2018
In the originally published version of this Article, the affiliation details for Dorina Avram incorrectly included “Department of Infectious Diseases and Immunology, College of Veterinary Medicine, University of Florida, 2015 SW 16th Ave, Gainesville, FL, 32608, USA”, instead of “UF Health Cancer Center, University of Florida, Gainesville, FL 32610, USA”. This has now been corrected in both the PDF and HTML versions of the Article.
References
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 (2011).
Zhu, J., Yamane, H. & Paul, W. E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Zhu, J., Guo, L., Watson, C. J., Hu-Li, J. & Paul, W. E. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J. Immunol. 166, 7276–7281 (2001).
Maier, E., Duschl, A. & Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 42, 2827–2833 (2012).
van Panhuys, N. et al. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc. Natl Acad. Sci. USA 105, 12423–12428 (2008).
Lee, G. R., Fields, P. E., Griffin, T. J. & Flavell, R. A. Regulation of the Th2 cytokine locus by a locus control region. Immunity 19, 145–153 (2003).
Koh, B. H. et al. Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma. Proc. Natl Acad. Sci. USA 107, 10614–10619 (2010).
Lee, G. R., Spilianakis, C. G. & Flavell, R. A. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nat. Immunol. 6, 42–48 (2005).
Cai, S., Lee, C. C. & Kohwi-Shigematsu, T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat. Genet. 38, 1278–1288 (2006).
Kim, K., Kim, N. & Lee, G. R. Transcription factors Oct-1 and GATA-3 cooperatively regulate Th2 cytokine gene expression via the RHS5 within the Th2 locus control region. PLoS One 11, e0148576 (2016).
Ansel, K. M. et al. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nat. Immunol. 5, 1251–1259 (2004).
Djuretic, I. M. et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 8, 145–153 (2006).
Naoe, Y. et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J. Exp. Med. 204, 1749–1755 (2007).
Kohu, K. et al. The Runx3 transcription factor augments Th1 and down-modulates Th2 phenotypes by interacting with and attenuating GATA3. J. Immunol. 183, 7817–7824 (2009).
Avram, D. et al. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J. Biol. Chem. 275, 10315–10322 (2000).
Avram, D., Fields, A., Senawong, T., Topark-Ngarm, A. & Leid, M. COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. Biochem. J. 368, 555–563 (2002).
Cismasiu, V. B. et al. BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. Oncogene 24, 6753–6764 (2005).
Cismasiu, V. B. et al. BCL11B participates in the activation of IL2 gene expression in CD4+T lymphocytes. Blood 108, 2695–2702 (2006).
Li, L., Leid, M. & Rothenberg, E. V. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science 329, 89–93 (2010).
Li, P. et al. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science 329, 85–89 (2010).
Ikawa, T. et al. An essential developmental checkpoint for production of the T cell lineage. Science 329, 93–96 (2010).
Wakabayashi, Y. et al. Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat. Immunol. 4, 533–539 (2003).
Albu, D. I. et al. BCL11B is required for positive selection and survival of double-positive thymocytes. J. Exp. Med. 204, 3003–3015 (2007).
Vanvalkenburgh, J. et al. Critical role of Bcl11b in suppressor function of T regulatory cells and prevention of inflammatory bowel disease. J. Exp. Med. 208, 2069–2081 (2011).
Albu, D. I. et al. Transcription factor Bcl11b controls selection of invariant natural killer T-cells by regulating glycolipid presentation in double-positive thymocytes. Proc. Natl Acad. Sci. USA 108, 6211–6216 (2011).
Uddin, M. N. et al. Transcription factor Bcl11b sustains iNKT1 and iNKT2 cell programs, restricts iNKT17 cell program, and governs iNKT cell survival. Proc. Natl Acad. Sci. USA 113, 7608–7613 (2016).
Avram, D. & Califano, D. The multifaceted roles of Bcl11b in thymic and peripheral T cells: impact on immune diseases. J. Immunol. 193, 2059–2065 (2014).
Zhang, S. et al. Antigen-specific clonal expansion and cytolytic effector function of CD8+T lymphocytes depend on the transcription factor Bcl11b. J. Exp. Med. 207, 1687–1699 (2010).
Abboud, G. et al. Transcription Factor Bcl11b controls effector and memory CD8 T cell fate decision and function during Poxvirus infection. Front. Immunol. 7, 425 (2016).
Califano, D. et al. Diverting T helper cell trafficking through increased plasticity attenuates autoimmune encephalomyelitis. J. Clin. Invest. 124, 174–187 (2014).
Walker, J. A. et al. Bcl11b is essential for group 2 innate lymphoid cell development. J. Exp. Med. 212, 875–882 (2015).
Yu, Y. et al. The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J. Exp. Med. 212, 865–874 (2015).
Califano, D. et al. Transcription factor Bcl11b controls identity and function of mature type 2 innate lymphoid cells. Immunity 43, 354–368 (2015).
Zhang, D. J. et al. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J. Immunol. 174, 6725–6731 (2005).
Reynolds, L. A., Filbey, K. J. & Maizels, R. M. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin. Immunopathol. 34, 829–846 (2012).
Kastner, P. et al. Bcl11b represses a mature T-cell gene expression program in immature CD4+CD8+thymocytes. Eur. J. Immunol. 40, 2143–2154 (2010).
Bailey, T. L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–208 (2009).
Tang, B. et al. Genome-wide identification of Bcl11b gene targets reveals role in brain-derived neurotrophic factor signaling. PLoS One 6, e23691 (2011).
Lotem, J. et al. Runx3-mediated transcriptional program in cytotoxic lymphocytes. PLoS One 8, e80467 (2013).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936 (2010).
Kojo, S. et al. Priming of lineage-specifying genes by Bcl11b is required for lineage choice in post-selection thymocytes. Nat. Commun. 8, 702 (2017).
Ebihara, T. et al. Runx3 specifies lineage commitment of innate lymphoid cells. Nat. Immunol. 16, 1124–1133 (2015).
Zhu, J. et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 5, 1157–1165 (2004).
Wei, G. et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35, 299–311 (2011).
Amsen, D., Antov, A. & Flavell, R. A. The different faces of Notch in T-helper-cell differentiation. Nat. Rev. Immunol. 9, 116–124 (2009).
Egawa, T. & Littman, D. R. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat. Immunol. 9, 1131–1139 (2008).
Egawa, T., Tillman, R. E., Naoe, Y., Taniuchi, I. & Littman, D. R. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J. Exp. Med. 204, 1945–1957 (2007).
Goodyear, A. W., Kumar, A., Dow, S. & Ryan, E. P. Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis. J. Immunol. Methods 405, 97–108 (2014).
Li, H. Toolkit for processing sequences in FASTA/Q formats https://github.com/lh3/seqtk/ (2013).
Andrews, S. Babraham bioinformatics - FastQC A. Quality control tool for high throughput sequence data http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Buenrostro, J. D. et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490 (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The Sequence alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
BroadInstitute. Picard tools http://broadinstitute.github.io/picard/ (2017).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Levanon, D. & Groner, Y. Structure and regulated expression of mammalian RUNX genes. Oncogene 23, 4211–4219 (2004).
Shao, Z., Zhang, Y., Yuan, G.-C., Orkin, S. H. & Waxman, D. J. MAnorm: a robust model for quantitative comparison of ChIP-Seq data sets. Genome Biol. 13, 1 (2012).
Tange, O. in Gnu parallel-the command-line power tool.; login: The USENIX Magazine 36, 42–47 (USENIX Association, California, 2011).
Acknowledgements
We gratefully acknowledge all the colleagues of the Center for Epigenetics at UF for insightful discussions on mechanisms of transcriptional regulation. We thank Dr. Yoram Groner and Dr. Dista Levanon for generously providing us with anti-Runx3 rabbit serum and Drs. Hiroyuki Hosokawa, Satoshi Hirose, and Ellen Rothenberg (California Institute of Technology) for their valuable suggestions on Bcl11b ChIP-seq. Support: NIH grants R01AI067846, R21AI112492, and UF Gatorade Trust and UF Cancer Center to Dorina Avram, T32AI007110 to K.J.L. and A.N.Z., R01DK105562 to L.Z., and USDA/ARS 8042-32000-104-00D to J.F.U.
Author information
Authors and Affiliations
Contributions
D.A. and K.J.L conceived and designed the experiments, interpreted the data and wrote the manuscript. K.J.L. performed HDM and helminth infection experiments and all DNA/RNA extraction, library prep, and subsequent sequencing for ChIP-seq, RNA-seq, and ATAC-seq. K.J.L., J.J.C., and A.N.Z. performed asthma and bone-marrow chimera experiments. X.L procured all experimental mice. J.F.U. provided all H3 larvae for infection and edited the manuscript. K.J.L., C.J., and R.G. performed processing of sequencing files and subsequent statistical analyses. K.J.L., D.A., J.J.C., L.Z., and M.P.K. analyzed and interpreted genomic data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorentsen, K.J., Cho, J.J., Luo, X. et al. Bcl11b is essential for licensing Th2 differentiation during helminth infection and allergic asthma. Nat Commun 9, 1679 (2018). https://doi.org/10.1038/s41467-018-04111-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-04111-0
This article is cited by
-
Human induced-T-to-natural killer cells have potent anti-tumour activities
Biomarker Research (2022)
-
PD-L1+ and XCR1+ dendritic cells are region-specific regulators of gut homeostasis
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.