Abstract
Hydrogenation is an effective way to tune the property of metal oxides. It can conventionally be performed by doping hydrogen into solid materials with noble-metal catalysis, high-temperature/pressure annealing treatment, or high-energy proton implantation in vacuum condition. Acid solution naturally provides a rich proton source, but it should cause corrosion rather than hydrogenation to metal oxides. Here we report a facile approach to hydrogenate monoclinic vanadium dioxide (VO2) in acid solution at ambient condition by placing a small piece of low workfunction metal (Al, Cu, Ag, Zn, or Fe) on VO2 surface. It is found that the attachment of a tiny metal particle (~1.0 mm) can lead to the complete hydrogenation of an entire wafer-size VO2 (>2 inch). Moreover, with the right choice of the metal a two-step insulator–metal–insulator phase modulation can even be achieved. An electron–proton co-doping mechanism has been proposed and verified by the first-principles calculations.
Similar content being viewed by others
Introduction
As a typical transition oxide, VO2 has a pronounced metal–insulator transition (MIT) behavior at the critical temperature near 68 °C, accompanying by a sharp resistance change up to five orders of magnitude and marked infrared switching effect within sub-ps time scale1,2,3,4,5. It has thus shown great potential for important applications in memory material6,7, smart window8,9 and ultra-fast optical switching device10. Many efforts have been devoted to improve the phase transition properties of metal oxides11,12,13,14,15,16, including the hydrogenation treatment, which has been demonstrated to be an effective way to tune the property of metal oxides17,18,19,20,21. Recent experiments observed that H-incorporations in M-VO2 could result in a very stable metallic phase at room temperature19,22, giving excellent thermoelectric performance23. Moreover, further injecting H into the lightly doped M-VO2 could create another insulating state at the heavily H-doping situation20, enabling the control of MIT in a reversible and consecutive manner. Previous studies examined the thermodynamic and kinetic properties of H or Li doping in VO2 lattice22,24,25, showing that H atoms preferred to diffuse along the c-axis of rutile VO2 or a-axis of monoclinic VO2. Although the hydrogenation techniques available are not sustainable as they are conventionally performed with noble-metal (Au, Pt, Pd) catalysts, high-temperature/pressure annealing treatment or high-energy proton implantation in vacuum condition22,26,27,28.
In this work, we report a facile approach to hydrogenate monoclinic VO2 film in acid solution at ambient condition by placing a low workfunction metal particle (Al, Cu, Ag, Zn, or Fe) on VO2 surface. The workfunction difference will cause electron flowing from metal particles to VO2, which in turn drives surrounding solution protons to penetrate into VO2 due to electrostatic attraction, resulting a stable H atoms doping. This process will not only stabilize the VO2 lattice in acid, but also induce the modulation of phase transitions under ambience conditions, which should be of great potentials for material applications. An electron–proton co-doping mechanism has been proposed and this synergetic doping method will stimulate more simple and cost-effective atomic doping techniques in the future.
Results
Metal-acid treatment induced H-doped VO2 film
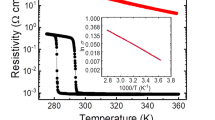
Is it possible to use acid solution as a natural proton source to achieve the hydrogenation of VO2 at ambient condition? At the first glance, this appears to be an impossible mission, as the textbook tells us that pristine metallic oxides including VO2 are easily dissolved in acid through the well-established reaction of VO2 + 4H+ → V4+ + 2H2O. Indeed, as shown in Fig. 1a, when a 30 nm M-VO2/Al2O3 (0001) epitaxial film grown by molecular beam epitaxy method29 (Supplementary Fig. 1) held by a plastic tweezers was put into a 2%wt H2SO4 acid solution, the yellowy VO2 epitaxial film completely disappeared within 3 h. Although when a steel tweezers was used to hold the sample, as shown in Fig. 1b, the same VO2 film suddenly demonstrated excellent anti-corrosion ability: it remains intact after 3 h in the acid solution. Scanning electron microscope (SEM) images in Fig. 1c show that the thickness and morphology of VO2 film hardly change even after 20 h in acid solution. In addition, the atomic force microscope (AFM) measurements show nearly zero thickness variation for metal-acid-treated samples (Supplementary Fig. 2a), which is consistent with the SEM cross-section image and confirms the anti-corrosion ability. More convincingly, the trace element analysis in Fig. 1d revealed that the concentration of V4+ cations in solution increased from 0.11 to 1.82 μg/ml after immersing a VO2 film held by a plastic tweezers in acid from 30 min to 20 h, whereas it kept very low value at 0.03–0.06 μg/ml with a steel tweezers. All these results firmly point to the fact that the attachment of a metal can give excellent anti-corrosion ability to VO2.
Metal-acid treatment induced hydrogenation of VO2 film. a The VO2 film on Al2O3 substrate held by a plastic tweezers was dissolved by 2%wt H2SO4 acid in 3 h. b Although a steel (Fe) tweezers attachment made the film intact in acid, showing pronounced anti-corrosion ability. c The SEM images for the cross-sections and surface morphologies of the VO2 film being treated by metal (Fe)-acid for 20 h, showing that the VO2 film maintains unchanged thickness and surface morphologies. The scale bar is 100 nm for the cross-sections and 500 nm for the surface morphologies, respectively. d Trace element analysis shows the V4+ concentrations in solution changing from 0.11 to 1.82 μg/ml after 30 min to 20 h with acid treatment, whereas very low V4+ concentration at 0.03–0.06 μg/ml is found at the same time period with metal (Fe)-acid treatment. The e XRD, f XPS, and g XANES characterizations for the pristine VO2 and metal (Fe)-acid-treated samples for 1.5 and 10 h, respectively. The pronounced (020) XRD peak shift from 39.8° to 36.7°, the increased V3+ and O–H XPS signals, and enhanced eg/t2g XANES signal ratio (reflecting the variation of electron occupancy) along with the increase of metal (Fe)-acid time, indicate the lattice changes and O–H bonds formations due to light and heavy hydrogenations
Interestingly, the hydrogenated VO2 produced by Au or Pd catalyst is found to be very stable in acid solution (Supplementary Fig. 2b). One can thus reasonably assume that the anti-corrosion ability of such metal-acid-treated VO2 is due to the hydrogenation. The X-ray diffraction (XRD) spectra in Fig. 1e show the dynamic shifts of (020) diffraction peak from 39.8° to 36.7° after the metal-acid treatment due to the cell expansion caused by H-incorporation, which agree well with the results for lightly and heavily hydrogenated VO2 through conventional noble-metal catalysis at high temperature (Supplementary Fig. 3a). These hydrogenated VO2 films show successive metallic and insulator states as the hydrogen doping concentration increasing30.
The X-ray photoelectron spectroscopy (XPS) measurements presented in Fig. 1f clearly indicate the conversion from V4+ to V3+ state as the result of H intercalation, which is further confirmed by the variations of O1s peak at ~531.6 eV for the O–H species. The change of valence state from V4+ to V(4−δ)+ or even to V3+ state is also verified by the X-ray absorption near-edge structure (XANES) spectra in Fig. 1g as the V L-edge curves shift continuously to lower energy. After the metal-acid treatment, the relative intensity ratio of the t2g and eg peaks in O K-edge curves decreased substantially, reflecting the variation of electron occupancy due to electron doping as well as the loss of the d// state upon hydrogenation31. All these spectroscopic features induced by metal-acid treatment for 1.5 and 10 h agree well with corresponding measurements on lightly and heavily hydrogenated VO2 through conventional catalysis techniques (Supplementary Fig. 3), respectively. It can thus be concluded that the metal-acid treatment can indeed create H-doping in the VO2 film.
Hydrogenation of a wafer-size VO2 film
It is noted that contact area between the metal tweezers and the VO2 film is actually quite small. To further quantitatively explore the effect of metal attachment, we have placed a tiny Cu particle (~1.0 mm in diameter) at the center of one 2-inch M-VO2/Al2O3(0001) epitaxial film, and immersed them together into 2%wt H2SO4 solution. It is observed in Fig. 2a that the bare VO2 film with yellowy color could be dissolved within 1.5–3 h. In sharp contrast, the small copper particle has provided the protection for the whole 2-inch wafer from acid corrosion. In addition, when the Cu particle is taken away after the treatment, the film remains stable in acid solution as it has already been hydrogenated (Supplementary Fig. 4).
Hydrogenation of a wafer-size VO2 film with a tiny Cu particle. a The two 2-inch VO2/Al2O3 wafers immersed in 2%wt H2SO4 acid solution. The sample with a tiny copper (Cu) particle (~1 mm) attached on the center surface exhibits pronounced anti-corrosion ability, whereas the bare VO2/Al2O3 film is completely corroded within 1.5–3 h, leaving the transparent Al2O3 substrate. The scale bar is 1 cm. b The resistance mapping for the 2-inch pristine VO2 film. c The resistance mapping for the metal(Cu)-acid-treated VO2. For the whole 2-inch wafer, the surface resistance is decreased by almost three orders of magnitude in comparison to the pristine film, reflecting the MIT of M-VO2 by hydrogenation. d The resistance measurement in air for the metal(Cu)-acid-treated M-VO2 as the function of heated temperature. Along with the pronounced hysteresis R–T curve, the metallic sample gradually recovers to the initial insulated M-phase VO2
A key evidence about the hydrogenation of VO2 is the insulator–metal transition at room temperature19,22, i.e., the hydrogenation converts the insulated M-VO2 to the metallic phase (Supplementary Fig. 5). In comparison with the original insulating M-VO2 film (Fig. 2b), the surface resistance of above Cu-acid-treated sample is decreased by almost three orders of magnitude (Fig. 2c). After cyclically heating the sample in air between 40 and 90 °C for about 2 h, the intercalated hydrogens could be completely removed, and the film is recovered back to the insulated phase (Fig. 2d), which is consistent with the results obtained from hydrogenated samples through conventional catalysis (Supplementary Fig. 6). From Fig. 2a, one can note that the small copper particle can be taken away to leave out pure H-doping material. It is certainly a much better approach than the conventional catalysis-based technique as those metal catalysts (Au or Pt) sputtered onto the VO2 film surface are hardly removable. In addition, the latter gives only limited hydrogenation area covered by catalysts (Supplementary Fig. 5b).
Hydrogenation effects controlled by different metals
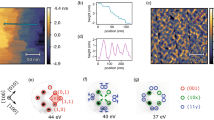
We have found that several other metals, such as Al, Cu, Ag, Zn, or Fe, can all induce hydrogenation and thereby protect VO2 from corrosion in acid, whereas Au and Pt can not. The effects of different metals are illustrated in Fig. 3a. One important parameter associated with the choice of the metal is the workfunction. The workfunction values calculated for the metals (Supplementary Fig. 7), VO2 and H0.5VO2 are plotted together with the reported experimental values32 in Fig. 3b. Simulations of pristine and hydrogenated VO2 systems were based on the most stable atomic models obtained by previous studies30. Because of high lattice symmetry, the electronic structure of H-doped VO2 is sensitive to the H-doping concentration but not to the atomic sites of H in lattice. By testing all of the 16 possible H-doping sites (Supplementary Fig. 8; Supplementary Table 1), we have taken the one with lowest energy for further investigation. A clear pattern can be observed: with the respect to the workfunction of VO2, the metal with smaller workfunction value can induce the hydrogenation. With such a workfunction difference, metals with higher electric Fermi level (EF) would donate electrons to the interfaced VO2 with lower EF (Fig. 3c). Calculations show that one (1 × 1) VO2 unit could extract 0.47–2.50e− from metals with lower workfunction (Fig. 3d; Supplementary Fig. 9; Supplementary Table 2). On the other hand, higher workfunction metals, Au and Pt, give nearly no extra electrons at the interface of M-VO2. It should also be noted in Fig. 3b that Al and Zn metals hold even lower workfunction than the lightly H-doped system of H0.5VO2, suggesting the continuing donation of electrons from metal to lightly hydrogenated VO2 which later attracts more hydrogen to penetrate. Therefore, the final products of Al/Zn-acid treatment are heavily H-doped VO2 with insulator phase while those of Ag/Cu-acid are conductive lightly H-doped VO2, as validated by XRD, XPS, XANES, and Raman characterizations in Supplementary Fig. 3. These results thus demonstrate that the electron-rich VO2 interface can attract and interact with the protons in acid solution, resulting in a feasible way to generate hydrogen atoms needed by hydrogenation.
Hydrogenation effects induced by different metals. a Metals, such as Al, Cu, Ag, can protect M-VO2 (1 cm × 1 cm size) from corrosion in 2%wt H2SO4 acid solution, whereas metals, Pt and Au, can not. b Computed and experimental workfunction (WF) values for metals, VO2, and lightly hydrogenated H0.5VO2, with the order of Pt > Au > VO2 > Cu > Ag > H0.5VO2 > Zn > Al. c Schematic depiction of electrons flowing from metal with a higher Fermi level (i.e., lower workfunction Wm) to semiconductor with a lower Fermi level (i.e., higher workfunction Ws) at the interface. d Computed differential charge distribution at Al/Cu/Pt–VO2(020) interfaces, showing that active metals (Al and Cu) donate effective electrons to VO2. Green and yellow bubbles represent hole and electron charges, respectively. Gray, red, cyan, brown, navy beads stand for V, O, Al, Cu, Pt atoms, respectively
By examining six VO2 surface sites for a proton to adsorb (inset graph in Fig. 4a and Supplementary Fig. 10), it is found that more doped electrons lead to higher adsorption energies for all sites (Fig. 4a). For instance, on site 1, the proton adsorption energy of 3.68 eV in neutral circumstance is increased to 5.04 eV for a VO2 unit with 4e− charge. The doped electrons also promote the diffusion of surface hydrogens into the VO2 crystal, with a possible migration pathway along the [100] direction (Supplementary Fig. 11). We can therefore propose an electron–proton co-doping strategy to create stable neutral H-doping in VO2. More specifically, driving by the electrostatic attraction, the surrounding protons could penetrate into VO2 to meet electrons, resulting in neutral H intercalation. The incorporation of H in the VO2 crystal prohibits further attack/adsorption of protons to oxygen, and increases the formation energy required for oxygen vacancy defect (Supplementary Fig. 12), resulting in the anti-corrosion ability in acid solutions.
Electron–proton co-doping mechanism. a Computed adsorption energies for a proton to six adsorption sites of VO2 (020) surface, increased with the increasing amount of doped electrons. b Evolutions of V-3d partial density of state (PDOS), suggest the change of semiconductor band gap in the insulated pristine VO2 to the zero energy gap in H0.25VO2. Fermi level is marked with purple dashed lines. c Computed differential charge distribution at H0.25VO2–VO2 interface, showing each H0.25VO2 supercell donated ~2.06e− to un-doped VO2. Here green and yellow bubbles represent hole and electron charges, respectively. d The schematic illustration of the contagious electron–proton co-doping mechanism with the metal-acid treatment to semiconductor: firstly the electrons flow to semiconductor when the metal contacts VO2 film. Once the metal/VO2 is immersing into acid solution, chemical reactions go sequentially as M – x[e−] → Mx+ and VO2 + x[e–] + x[H+] → H x VO2. Here protons penetrate to meet electrons, creating conductive H-doped structure. Meanwhile the attached metal (M) is gradually dissolved in acid to become M+ cations for balancing charges in solution. Then the electrons flow from conductive H-doped structure to the un-doped parts, driving more proton penetration. Finally the repeated electron flowing–proton penetration–phase transition–electron further flowing cycle expands toward full H-doping
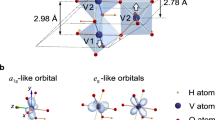
The H-doping changes the VO2 electronic structures. For a VO2 unit with small H-doping concentration of H0.25VO2 (Fig. 4b), the evolution of the electronic structure is reflected by the computed partial density of state (PDOS) of the V-3d orbitals in Fig. 4b. The formation of H–O bonds causes electrons transferring from H to O atoms (Supplementary Table 3), which in turn promotes the electron occupancy of V-3d orbitals. Such effects give rise to the up-shifting of Fermi level from the pure VO2 to H0.25VO2 (Fig. 4b). Originally, VO2 exhibits a typical insulating state, with wide energy gap consisting of fully occupied valence band and empty conduction band. The H-doping then makes the conduction band edge states partially occupied, as for the case of H0.25VO2.
The same concept can be also used to explain the contagious hydrogenation process that enables a ~1 mm metal particle to convert a 2-inch semiconductor wafer. As shown in Fig. 3b, the work functions of the lightly hydrogenated H0.25VO2 with three facets of (020), (011), (100) are 4.32–4.96 eV, which are all lower than those of pristine VO2 around 5.12–5.85 eV. For any H-doped VO2 parts created by metal-acid treatment, electrons would flow/dope into neighboring unhoped VO2 with lower Fermi level (Fig. 4c; Supplementary Fig. 13). The electron-rich area drives further proton penetration to the neighboring un-doped VO2.
Electron–proton co-doping mechanism
A contagious spreading of electron–proton co-doping mechanism is summarized in Fig. 4d: firstly the metal with lower workfunction donates electrons to the interfaced VO2 due to Fermi level difference, resulted in extra electrons accumulated in oxide layer; Then the doped electrons attract surrounding protons in acid solution to penetrate into the oxide semiconductor, creating H-doped structure at the top layer and causing the surface insulator-to-metal phase transition. Simultaneously, the attached metal particle is partially dissolved in acid, which balances the total charge in solution. This balance of charge is essential to drive the hydrogenation of VO2 as this route:
Otherwise, the reaction will follow the route of VO2 + 4H+ → V4+ + 2H2O, resulting the corrosion of VO2 in acid. The test of immersing only parts of a Cu/VO2 sample into acid without metal in solution causes no anticorrosive property (Supplementary Fig. 14), clearly showing this situation. After the above stage, the conductive H-doped structures delivery electrons to adjacent un-doped VO2 parts, triggering the next round of electron–proton co-doping and insulator-to-metal phase transition. Finally, the repeated electron flowing–proton penetration–phase transition–electron further flowing cycle expands toward full H-doped oxide material. This spreading of co-doping from metal center to edge is reflected by the onion-like contour map of resistance after metal-acid treatment (Fig. 2c), which decreases gradually from the metal-attached center to the wafer edge. In addition, the hydrogenation process is also gradually completed from the top to bottom layer with considering the time-dependent hydrogenation-related Raman or XRD signals (Supplementary Fig. 15).
It should be pointed out that since the corrosion of VO2 caused by oxygen atom moving out of lattice is much slower than the migrations of electrons or protons, the dynamics of this co-doping mechanism ensure the quick hydrogenation of VO2 surface before being corroded by acid, resulting in the anti-corrosion property of wafer-size VO2 film even at the beginning stage since we found the distinct resistance decrease of VO2 with several seconds metal-acid treatment.
Discussion
On the basis of the proposed concept, one can anticipate that the metals with very low workfunction, such as Al or Zn, can lead to heavy hydrogenation of VO2 films in acid solution. It means that the induced metallic state would eventually be transferred into another new insulating state because of nearly saturated hydrogenation (Supplementary Fig. 3; Supplementary Fig. 16), which agree well with the different H concentrations revealed by secondary-ion mass spectrometry measurement (Supplementary Fig. 17). This observation is consistent with very recent findings of the consecutive insulator–metal–insulator transitions induced by increasing H-doping concentration20. In addition, based on this metal-acid treatment, partially hydrogenation process with a selected region can also be easily achieved by control the immersing area in acid solution (Supplementary Fig. 18). Remarkably, this simple metal-acid treatment is found to be a universal strategy that can be extended to doping ions in general. Replacing the acid solution by polymeric solution with Li+, metallic Li-doped VO2 films can also be obtained (Supplementary Fig. 19).
The ability to hydrogenate VO2 with protons in acid solution demonstrated here provides a facile strategy to induce phase modulation of VO2 materials, and the later successful doping of Li+ into VO2 suggests a general atomic doping approach of using proton or cation solvent sources together with electrons from metals. It is a sustainable approach that operates at ambient condition in an environment friendly manner by completely avoiding the use of precious catalysts and high-energy consumptions. The doping concept established in this study will have strong impact on the development of new functional materials in different applications.
Methods
Thin-film growth
The 2-inch wafer-size VO2 (020) epitaxial films were grown on c-cut sapphire by an rf-plasma assisted oxide molecular beam epitaxy (rf-OMBE) equipment and more details for the film preparation are reported elsewhere29.
Conventional hydrogenation treatment for VO2 film
Nano-sized Au islands were sputtered on M-VO2 surface as the catalysis. The Au/VO2 samples were annealed in tube furnace with the forming gas (15% H2/85% Ar) under various conditions. The lightly doped metallic H-VO2 (120 °C for 2 h) and heavily doped insulated H-VO2 (180 °C for 10 h) were prepared.
Characterizations
The resistance as the function of temperature was examined by Keithley 2400 sourcemeter with a variable temperature stage. For all of the measurements, the temperature sweeping rate was set at 0.1 K/s; The resistance distribution mapping for the prepared wafer-size VO2 film were tested on room temperature and 120 °C, respectively. The cross-section and surface morphologies were investigated by Scanning Electron Microscopy (Gemini Fe-SEM 500 and FEI Sirion 200). Raman spectra were recorded by an integrated laser Raman system (LABRAM HR, Jobin Yvon). The 632.8 nm He–Ne laser was used as the excitation source. To obtain direct information about the hydrogen concentration of the metal-acid VO2 film sample, the secondary-ion mass spectrometry (SIMS) measurements (Quad PHI6600) were conducted. The ICP-AES equipment (Optima 7300DV) was used to trace element concentration. The wavelength of Cu, V adopted 327.393, 290.880 nm respectively. The emission power is 1250 w. The distinguishability reached to 0.003 nm at 200 nm, and the detection limit is 4 ng/ml.
Synchrotron-based measurements
Synchrotron X-ray diffraction spectra, including θ − 2θ, X-ray reflectivity (XRR), Φ-scan, rocking-curve, were conducted at the BL14B beamline of the Shanghai Synchrotron Radiation Facility (SSRF). The SSRF is a third-generate accelerator with a 3.5 GeV storage ring. The BL14B beamline shows the energy resolution (ΔE/E) of 1.5 × 10−4 @10 keV and the beam size of 0.3 × 0.35 mm with the photo flux of up to 2 × 1012 phs/s@10 keV. Considering the photo flux distribution and the resolution, the 0.12398 nm X-ray was chosen during the experiment.
The X-ray absorption near-edge spectroscopy (XANES) was conducted at the XMCD beamline (BL12B) in National synchrotron radiation laboratory (NSRL), Hefei. The total electron yield (TEY) mode was applied to collect the sample drain current under a vacuum better than 3.75 × 10−10 Torr. The energy range is 100–1000 eV with an energy resolution of ca. 0.2 eV. The X-ray incident angle is 54.7°. During the measurement, the samples were firmly adhered on the conductive substrate with random orientation, so the polarization dependence is not considered.
The X-ray photoelectron spectroscopy (XPS) beamline was conducted in National synchrotron radiation laboratory (NSRL), Hefei. The photoemission beamline covers photon energies from 100 to 1000 eV with a typical photon flux of 1 × 1010 phs/s and a resolution (E/ΔE) better than 1000 at 244 eV. The analysis chamber is connected to the beamline and equipped with a VG Scienta R3000 electron energy analyzer, and the base pressure is 1.5 × 10−10 Torr.
First-principles calculations
All calculations are performed with density functional theory (DFT), using the Vienna ab initio simulation package (VASP) code33. The exchange and correlation terms are described using general gradient approximation (GGA) in the scheme of Perdew–Burke–Ernzerhof (PBE)34. Core electrons are described by pseudopotentials generated from the projector augmented-wave method35, and valence electrons are expanded in a plane-wave basis set with an energy cutoff of 480 eV. Slab model method is used to model the VO2 surface and metal–VO2 interface, the thickness of vacuum are larger than 15 Å. The DFT+U method is employed to optimize the structure, U and J are chosen to be 4 and 0.68 eV. The geometry relaxation is carried out until all forces on the free ions are converged to 0.01 eV/Å. In the calculation of electronic structures, DFT with hybrid functionals proposed by Heyd, Scuseria, and Ernzerhof (HSE06) is used36. Climbing image nudged elastic band (CI-NEB) method37 is used to find the minimum energy paths and the transition states for diffusion of H from surface to subsurface, with a force converge <0.05 eV/Å.
Data availability
The remaining data contained within the paper and supplementary files are available from the authors upon request.
References
Park, J. H. et al. Measurement of a solid-state triple point at the metal–insulator transition in VO2. Nature 500, 431–434 (2013).
Lee, S. et al. Anomalously low electronic thermal conductivity in metallic vanadium dioxide. Science 355, 371–374 (2017).
Qazilbash, M. M. et al. Mott transition in VO2 revealed by infrared spectroscopy and nano-imaging. Science 318, 1750–1753 (2007).
Morrison, V. R. et al. A photoinduced metal-like phase of monoclinic VO2 revealed by ultra-fast electron diffraction. Science 346, 445–448 (2014).
O’Callahan, B. T. et al. Inhomogeneity of the ultra-fast insulator-to-metal transition dynamics of VO2. Nat. Commun. 6, 6849 (2015).
Vardi, N. et al. Ramp-reversal memory and phase-boundary scarring in transition metal oxides. Adv. Mater. 29, 1605029 (2017).
Driscoll, T. et al. Memory metamaterials. Science 325, 1518–1521 (2009).
Kim, H. et al. Flexible thermochromic window based on hybridized VO2/graphene. ACS Nano 7, 5769–5776 (2013).
Gao, Y. et al. VO2–Sb:SnO2 composite thermochromic smart glass foil. Energy Environ. Sci. 5, 8234 (2012).
Hilton, D. J. et al. Enhanced photosusceptibility near Tc for the light-induced insulator-to-metal phase transition in vanadium dioxide. Phys. Rev. Lett. 99, 226401 (2007).
Aetukuri, N. B. et al. Control of the metal–insulator transition in vanadium dioxide by modifying orbital occupancy. Nat. Phys. 9, 661–666 (2013).
Cao, J. et al. Strain engineering and one-dimensional organization of metal–insulator domains in single-crystal vanadium dioxide beams. Nat. Nanotechnol. 4, 732–737 (2009).
Fan, L. L. et al. Strain dynamics of ultrathin VO2 film grown on TiO2 (001) and the associated phase transition modulation. NanoLetters 14, 4036–4043 (2014).
Budai, J. D. et al. Metallization of vanadium dioxide driven by large phonon entropy. Nature 515, 535–539 (2014).
Jeong, J. et al. Suppression of metal–insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science 339, 1402–1405 (2013).
Nakano, M. et al. Collective bulk carrier delocalization driven by electrostatic surface charge accumulation. Nature 487, 459–462 (2012).
Lu, N. et al. Electric-field control of tri-state phase transformation with a selective dual-ion switch. Nature 546, 124–128 (2017).
Zhou, Y. et al. Strongly correlated perovskite fuel cells. Nature 534, 231–234 (2016).
Wei, J., Ji, H., Guo, W., Nevidomskyy, A. H. & Natelson, D. Hydrogen stabilization of metallic vanadium dioxide in single-crystal nanobeams. Nat. Nanotechnol. 7, 357–362 (2012).
Yoon, H. et al. Reversible phase modulation and hydrogen storage in multivalent VO2 epitaxial thin films. Nat. Mater. 15, 1113–1119 (2016).
Elias, D. C. et al. Control of graphene’s properties by reversible hydrogenation: evidence for graphane. Science 323, 610–613 (2009).
Filinchuk, Y. et al. In situ diffraction study of catalytic hydrogenation of VO2: stable phases and origins of metallicity. J. Am. Chem. Soc. 136, 8100–8109 (2014).
Wu, C. et al. Hydrogen-incorporation stabilization of metallic VO2(R) phase to room temperature, displaying promising low-temperature thermoelectric effect. J. Am. Chem. Soc. 133, 13798–13801 (2011).
Lin, J. et al. Hydrogen diffusion and stabilization in single-crystal VO2 micro/nanobeams by direct atomic hydrogenation. NanoLetters 14, 5445–5451 (2014).
Kulish, V. V., Koch, D. & Manzhos, S. Ab initio study of Li, Mg and Al insertion into rutile VO2: fast diffusion and enhanced voltages for multivalent batteries. Phys. Chem. Chem. Phys. 19, 22538–22545 (2017).
Shi, J., Zhou, Y. & Ramanathan, S. Colossal resistance switching and band gap modulation in a perovskite nickelate by electron doping. Nat. Commun. 5, 4860 (2014).
Chen, X., Liu, L., Yu, P. Y. & Mao, S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011).
Teschner, D. et al. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 320, 86–89 (2008).
Fan, L. L. et al. Growth and phase transition characteristics of pure M-phase VO2 epitaxial film prepared by oxide molecular beam epitaxy. Appl. Phys. Lett. 103, 131914 (2013).
Chen, S. et al. Sequential insulator–metal–insulator phase transitions of VO2 triggered by hydrogen doping. Phys. Rev. B 96, 125130 (2017).
Quackenbush, N. F. et al. Nature of the metal–insulator transition in ultrathin epitaxial vanadium dioxide. NanoLetters 13, 4857–4861 (2013).
Michaelson, H. B. The workfunction of the elements and its periodicity. J. Appl. Phys. 48, 4729–4733 (1977).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Erratum: “Hybrid functionals based on a screened Coulomb potential” [J. Chem. Phys. 118, 8207 (2003)]. J. Chem. Phys. 124, 219906 (2006).
Sheppard, D., Xiao, P., Chemelewski, W., Johnson, D. D. & Henkelman, G. A generalized solid-state nudged elastic band method. J. Chem. Phys. 136, 074103 (2012).
Acknowledgements
This work was partially supported by the National Basic Research Program of China (2014CB848900), the National Key Research and Development Program of China (2016YFA0401004), the National Natural Science Foundation of China (U1432249, 11574279, 11404095, 21633006, 11704362), the Fundamental Research Funds for the Central Universities; the funding supported by the Youth Innovation Promotion Association CAS and the Open Research Fund of State Key Laboratory of Pulsed Power Laser Technology, Electronic Engineering Institute. We also acknowledge supports from the X-ray diffraction beamline (BL14B1) in Shanghai Synchrotron Radiation Facility, the XMCD beamline (BL12B) and photoelectron spectroscopy beamline (BL10B) in National Synchrotron Radiation Laboratory (NSRL) of Hefei.
Author information
Authors and Affiliations
Contributions
Y.C., J.J. and C.Z. conceived the study. Y.C. and C.Z. designed the experiment and performed the initial tests. Z.W. and J.J. conducted the theoretical calculations. Y.C., S.C., H.R., L.W., G.Z. and Y.L. conducted the synchrotron-based measurements. Y.C., J.J., C.Z. and Y.L. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Wang, Z., Chen, S. et al. Non-catalytic hydrogenation of VO2 in acid solution. Nat Commun 9, 818 (2018). https://doi.org/10.1038/s41467-018-03292-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-03292-y
This article is cited by
-
Spatially-resolved insulator-metal transition for rewritable optical gratings
Communications Materials (2021)
-
Long-range propagation of protons in single-crystal VO2 involving structural transformation to HVO2
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.