Abstract
The Cambrian explosion, the rapid appearance of most animal phyla in the geological record, occurred concurrently with bottom seawater oxygenation. Whether this oxygenation event was triggered through enhanced nutrient supply and organic carbon burial forced by increased continental weathering, or by species engaging in ecosystem engineering, remains a fundamental yet unresolved question. Here we provide evidence for several simultaneous developments that took place over the Ediacaran–Cambrian transition: expansion of siliceous sponges, decrease of the dissolved organic carbon pool, enhanced organic carbon burial, increased phosphorus removal and seawater oxygenation. This evidence is based on silicon and carbon stable isotopes, Ge/Si ratios, REE-geochemistry and redox-sensitive elements in a chert-shale succession from the Yangtze Platform, China. According to this reconstruction, sponges have initiated seawater oxygenation by redistributing organic carbon oxidation through filtering suspended organic matter from seawater. The resulting increase in dissolved oxygen levels potentially triggered the diversification of eumetazoans.
Similar content being viewed by others
Introduction
The Cambrian bioradiation is thought to have occurred when dissolved oxygen concentrations in seawater exceeded a critical threshold of ~5–20 µmol l−1, i.e., 2–7 percent of present surface seawater1. Rising oxygen levels were thus an important factor for the development of animals on Earth2. Ultimately, atmospheric and seawater dissolved oxygen levels increase during the growth of the continental organic carbon reservoir because over > Myr timescales, the CO2 flux into the atmosphere–ocean system is balanced by carbon burial which releases oxygen3. Suggested triggers for ocean oxygenation during the Ediacaran–Cambrian transition include increased weathering fluxes4,5,6,7 that would cause an increase in oceanic primary production and organic carbon burial5, 6. Enhanced clastic sedimentation during times of high tectonic activity reduces oxidation of organic carbon and causes rising oxygen levels4.
Purely biogenic mechanisms might have been responsible for the Ediacaran–Cambrian oxygenation too. A possible terrestrial biogenic trigger of oxygenation is the biological enhancement of phosphorus supply to the ocean by weathering in the Neoproterozoic that resulted in a rise of atmospheric oxygen8. A possible terrestrial driver is the development of the fungi-lichen ecosystem that led to the formation of an organic-rich, upper soil layer. This layer restricted the consumption of increased atmospheric oxygen required to weather the subsoil regolith9. In the marine realm, a potential trigger of seawater oxygenation was presented in the evolution and expansion of metazoans themselves: some evolutionary innovations would have increased the export of organic matter to the deep sea, and lowered the oxygen demand in shallow water10. Suggested mechanisms include an increased efficiency of the biological pump by the evolution of large eukaryotes and rapidly sinking fecal pellets10, or the evolution of eumetazoa, enhancing organic carbon transfer to sediment and reducing oxygen consumption in the overlying water column11,12,13,14. A recent and thus far untested hypothesis states that metazoan development itself caused seawater oxygenation by mechanisms of ecosystem engineering12. One proposed mechanism is that benthic filter feeding by sponges has led to seawater oxygenation12. By removing large amounts of dissolved and fine particulate organic carbon from seawater, the respiratory oxygen demand was shifted to a greater depth12, 13. The ensuing increase in seawater dissolved oxygen concentrations led to enhanced phosphorus burial and P sequestration by sponge symbionts15, further decreased primary productivity, and thus led to lower oxygen demands and higher dissolved oxygen levels12.
It is challenging to investigate the role of sponges for seawater oxygenation because their impact on the environment depends on their abundance. Unfortunately, because of incomplete sponge spicule preservation, sponge abundance cannot be directly obtained from the Precambrian–Cambrian fossil record. Essentially, all pre-Cenozoic biosiliceous material has been diagenetically altered, with chert (microcrystalline quartz) being the ultimate product of the diagenetic pathway. Cherts are near-ubiquitous in Ediacaran–Cambrian boundary sections, and the presence of sponge spicules suggests that the silicon stems at least partially from the dissolution and reprecipitation of siliceous sponge spicules. Silica diagenesis might even completely obliterate fossil evidence for siliceous sponges, and would have caused the 240-million-year lag time between the origin of silicean spicules (estimated from molecular clock analysis16) and their unequivocal identification in the fossil record. Chert occurrence, petrology and geochemistry thus need to be invoked to reconstruct sponge abundance. However, radiolarians17, 18, inorganic precipitation of silica from hydrothermal fluids19 and seawater20, 21 are all potential Si sources for cherts from the Ediacaran–Cambrian boundary, complicating interpretations. The recent demonstration that siliceous sponges often have distinctly different Si stable isotope ratios22, 23 implies that siliceous sponge abundance can be reconstructed using Si stable isotopes—provided that the bulk Si stable isotope composition was not altered.
Here we explore one of the most complete stratigraphic sections from the slope-to-basin setting at the SE margin of the Yangtze Platform to determine the abundance of siliceous sponges and to evaluate their biogeochemical impact during the Ediacaran–Cambrian transition. We show that the silicon sources of cherts can be constrained by a multi-proxy approach employing Si stable isotopes, Ge/Si ratios and rare earth elements (REE). The relative abundance of siliceous sponge spicules can be reconstructed using Si stable isotopes. This approach overcomes the challenge of incomplete spicule preservation that has so far impeded testing the hypothesis of seawater oxygenation by (siliceous) sponge proliferation12. We determine local changes in the oxidation states of seawater and sediment using Ce-anomalies and enrichment factors (EFs) of redox-sensitive trace metals. We derive variations in the DOC pool size from Ge/Si of clays and Y/Ho ratios. Our data suggests that siliceous sponges with low oxygen demands24 expanded on the continental slope across the Ediacaran–Cambrian boundary. These expanding sponge communities shifted the respiratory oxygen demand from the water column to the water-sediment interface by organic matter filter feeding. The resulting shift in oxygen demand to greater water depth led to increased P burial, resulting in lowered primary productivity and thus lowered oxygen consumption.
Results
Geological setting and samples
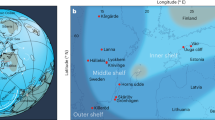
The Lijiatuo section is an almost completely exposed section on the Yangtze Platform, Hunan Province (Fig. 1a) that straddles the Ediacaran–Cambrian boundary. Recent evidence from litho- and chemostratigraphy25, as well as U-Pb age constraints26 allow for the integration of the Lijiatuo section into the Ediacaran–Cambrian stratigraphic framework of the Yangtze Platform25, placing its depositional history between ~550 and 525 Ma. The Lijiatuo section comprises the Liuchapo Fm. and the lower part of the Xiaoyanxi Fm., both representing deep-water depositional environments below storm wave base at the continental slope to basin. The sedimentary facies change from mostly pure chert to organic-matter-rich siliceous shales (Fig. 1b). The bottom of the section is dominated by black, bedded cherts with total organic carbon (TOC) concentrations <1 wt%, where the upper part is characterized by mostly phosphatic-siliceous shales and finely laminated organic-rich black shales with TOC concentrations of up to 15.1 wt% (Supplementary Data 1). The predominant minerals are quartz and illite. Some samples contain > 5 wt% barite. Pyrite, K-feldspar, Ba-feldspar and an unspecified 15 Å clay mineral occur locally as accessory phases (Supplementary Data 2). The lithostratigraphic and formational boundary is composed of phosphate-nodule bearing siliceous rocks and organic-rich shales. These shales are partly enriched in redox-sensitive elements (Supplementary Data 3), such as in the Ni and Mo-rich layer27 that is regionally exposed in the lowermost black shales of the Xiaoyanxi Fm. on the Yangtze Platform. Despite an overall low fossil content, fossil sponges or sponge spicules are present in both formations28, with abundant sponge spicules in some layers, particularly in the Xiaoyanxi Fm. Some samples from the Liuchapo Fm. contain spherical objects, i.e., putative radiolarians.
Lithostratigraphic profile of the Lijiatuo section and C- and Si stable isotope ratios of chert and siliceous shales and the paleogeographic setting of the site. The Lijiatuo section a is located in the continental slope-to-basin setting at the Yangtze Platform b and contains chert, siliceous shales and regional, Lower Cambrian marker horizons, i.e., phosphate bearing units (Phos) and a Ni-Mo layer as well as an ash layer (Ash) dated to 546 ± 12 Ma26. The sedimentary facies of the section changes towards higher TOC concentrations as shown by symbol colour (blue: <5 wt%, red: 15 wt%) as well as towards higher the clay-mineral contents (represented by the Al2O3 concentration, increasing symbol size). Scale bar, 10 m
Geochemical data
The silicon stable isotope ratio decreases from 1.1‰ δ 30Si at the base of the section to −0.5‰ δ 30Si at its top (Supplementary Data 4). The sedimentary sponge spicule abundance, calculated from δ30Si values (see Methods) (Fig. 2b) shows an overall increasing trend across the Lijiatuo section. Likewise, the relative sponge spicule abundance, fsponge, defined as the sponge-derived Si relative to the sum of Si derived from non-detrital components (cf. Methods) (yellow shade in Fig. 2c–f) shows an overall increasing trend across the Lijiatuo section. TOC concentrations vary along with fsponge (Fig. 2c). CeN/Ce*N is ~1 at the base of the section and drops to values as low as 0.42 and overall follows variations in fsponge (Fig. 2d). Ge/Si(illite) (see Methods) decrease in the lowermost 20 m of the section from > 50 to <5 and remain relatively constant above. Similarly, YN/HoN ratios initially decrease from 2.1 to 1.0 and mostly remain below 1.5 (Fig. 2f).
The estimated siliceous sponge spicule abundance and geochemical records across the Ediacaran–Cambrian boundary at the Lijiatuo section. a Stratigraphic context and age of the Lijiatuo section based on stratigraphic records from South China25(*) and a dated ash layer26(**); b Abundance of Si-bearing components calculated by a geochemical mass balance. Colour shading at the bottom indicates several estimates for the abundance of sponge-derived- and inorganic silica based on assumptions of ε30Si(sponge-seawater) between −6‰ and −2‰. Occurrences of sponge spicules and putative radiolarians, i.e., round objects (Supplementary Fig. 5) are indicated by black circles (our samples) and gray circles28. c TOC concentrations; d CeN/CeN* (Ce-anomalies); open symbols mark samples with PrN/YbN > 1, indicating flat, non-seawater REE patterns; e Al2O3-normalized Ge/Si and corresponding Ge/Si of illite; f YN/HoN ratios. For better visibility, samples are plotted with an equidistant X axis. In c-f fsponge (yellow shades) shows the abundance of Si in sponges relative to total non-detrital Si-bearing components that is independent of ε30Si(sponge-seawater). Uncertainty envelopes of fsponge are calculated from uncertainties in endmember estimates and a Monte Carlo procedure, and represent 50% confidence intervals (Supplementary Data 6)
The primary silicon isotope signature of chert
The silicon isotope record of cherts and siliceous shales can provide information about silicon sources provided that the isotopic signature has not been significantly modified during early diagenesis of sedimentary opal (i.e., dissolution of some fraction of the biogenic Si) and late diagenesis when silica phase transformations occur.
Chert precursors, such as siliceous sediments can shift in their bulk silicon isotope ratios during early diagenesis by selective dissolution in silicon-poor water29. However, this process was subdued in the Precambrian when seawater silicon concentrations were close to the opal-A saturation at ~28 p.p.m. Si30. Sponges and radiolarians were the first organisms of sufficient abundance to utilize silicon. Upon their first appearance seawater silicon concentrations were still high31, and are thought to have remained close to these levels, as suggested by the absence of an increase in bulk chert δ30Si over ~ 25 Myrs (see discussion below). Thus, dissolved silicon concentration remained high until the early to middle Paleozoic radiation of sponges and radiolarians32. Dissolved silicon high in concentration may even have diffused into sediments and led to inorganic opal-CT precipitation30, or to microbially mediated opal-A precipitation33. The high silicon concentration in seawater thus hampered early diagenetic Si loss from Precambrian and Paleozoic marine sediments and ensured that the primary Si stable isotope signature of the deposited siliceous sediments was not shifted during early diagenesis.
Exchange of silicon during diagenetic phase transformation (from opal-A to opal-CT to quartz) can potentially affect the silicon isotope composition of chert. Geilert et al.34 recently demonstrated that opal-CT in fossil volcanic sinter deposits was systematically 1.3‰ lower in δ30Si than opal-A sinter deposits from the same location. This observation suggests substantial 30Si-loss during opal-A to opal-CT transformation. An alternative and still untested explanation for this observation, however, is that the initial δ30Si value differed between these two phases. We suggest that the high-SiO2 siliceous sediments studied here have been resistant to Si stable isotope shifts during diagenesis. One reason is a simple mass balance consideration: to shift the bulk chert δ30Si requires unrealistically high mass losses or Si exchange, given that Si is the major constituent of the rock. Another is that during silica phase transformations, the exchange of Si between sedimentary silica layers with high and variable clay mineral contents is predicted to be negligible. Firstly, exchange of fluids between sediment layers is not favoured by the presence of impervious clay-rich layers. Secondly, the kinetics and hence burial depth of the silica phase transformations depend on detrital mineral concentrations35. This means that a stack of siliceous sediment with variable detrital mineral contents transforms to higher silica polymorphs (opal-CT and quartz) with different rates during burial. The ensuing differences in the timing of silica phase transformations impede the exchange of Si between sediment layers. These conditions act in favor of preserving the primary bulk silicon isotope composition of siliceous sediments deposited in siliciclastic depositional environments. This inference is supported by the preservation of isotopic heterogeneity over centimeter- to decimeter scales in our samples. Dissolution-reprecipitation will, however, affect silicon isotope ratios at the microscale36. We conclude that any diagenetic shift in the silicon isotope composition of our samples is likely negligible and that bulk rock silicon isotope ratios can be interpreted in terms of paleo-environmental conditions.
Seawater and siliceous sponge spicules as silicon source
Silicon isotopes, REE patterns, Ge/Si ratios, and petrographic evidence suggest that inorganic precipitates from seawater and siliceous sponge spicules are the dominant Si constituents. In contrast, the fraction of Si hosted by clay minerals and detrital quartz is variable and lower (mostly < 50%).
High δ30Si values at the sections’ base (Fig. 1b) suggest inorganic silica precipitation from seawater, in line with previously reported δ30Si values from Ediacaran inorganic cherts21. The oceans’ dissolved silicon is dominantly supplied by rivers37 that carry silicon depleted in 28Si by clay formation during continental weathering38. Modern rivers thus carry dissolved Si with an average δ30Si value of 1.28‰39. Moving upsection, the trend towards lower δ30Si suggests a progressive contribution of silica from siliceous sponges (and possibly radiolarians, as discussed below) that contain silicon with a low δ30Si signature22, 23. The Si stable isotope fractionation in modern sponges is thought to be controlled by Si influxes into and effluxes out of sclerocyte cells where Si isotopic fractionation, denoted by ε30Si (ε = α−1) attains a constant value at high seawater dissolved Si concentrations22, 23. We thus assume a constant ε30Si(sponge-seawater) for Precambrian sponges, because sclerocyte cells existed already before 580 Ma40 and seawater had a high Si concentration30, 32.
We can discount an increasing contribution of 28Si-rich hydrothermal silicon upsection based on REE patterns and Ge/Si. REEs, scavenged from seawater into authigenic phases, consistently show seawater-like patterns with Eu/Eu* ~ 1, variably negative Ce anomalies and YN/HoN > 1 (Supplementary Fig. 1 and Supplementary Data 5). The Ge/Si ratios of pure chert samples range between 0.4 and 0.8 µmol mol−1 (Supplementary Fig. 2 and Supplementary Data 3), similar to Precambrian seawater silica precipitates at 0.25–0.8 µmol mol−1 21 and modern seawater at 0.7 µmol mol−1, but much lower than hydrothermal fluids at ~7 µmol mol−1 41.
We can also discount that the trend in decreasing δ30Si (cf. Supplementary Data 4) is caused by clay mineral or possible TOC abundance-controlled variations in the Si stable isotope fractionation factor for silica precipitation (Supplementary Note 1). This independence is supported by the constant δ30Si values for multiple bulk chert samples from within two stratigraphic layers with variable TOC and Al2O3 concentrations (Supplementary Fig. 3). We note that 28Si could potentially be enriched in sediment through preferential 28Si adsorption onto Fe-Mn oxyhydroxides. Reactive Fe-Mn surfaces might be responsible for the sedimentary trace metal enrichment (see discussion below). This effect however cannot explain the δ30Si decreases in the lowermost part of the section where trace elements are not enriched. No correlation between trace element enrichment and δ30Si can be observed (see Supplementary Fig. 4).
An underlying assumption of our Si isotope mass balance is that the oceanic Si inventory was sufficiently large30, such that neither its Si concentration nor its δ30Si changed due to biogenic silica precipitation. This assumption is supported by the continuous decrease in δ30Si values over a period of ~25 Myrs along the entire section. If biogenic silica precipitation were significant relative to that of inorganic silica δ30Si would be expected to transiently increase. If the output flux of silica through siliceous sponges had been constant and high, in contrast, seawater would attain a lower silicon concentration and a higher steady state δ30Si value. As a consequence, the δ30Si of sponge spicules would increase within a few million years and bulk chert δ30Si would increase along the section. We conclude that only the abundance of siliceous sponge spicules and potentially some minor radiolarians are the cause of the low δ30Si Si in chert and siliceous shales, as supported by petrographic evidence for sponge spicules and putative radiolarians (Fig. 2b and Supplementary Fig. 5). Therefore, the shift in δ30Si allows for the determination of sedimentary sponge spicule abundance by mass balance (see Methods).
We observe that the relative abundance of sponge spicules, fsponge, defined as sponge-derived Si relative to the sum of Si derived from non-detrital components (cf. Methods), steadily increases throughout the Ediacaran–Cambrian Liuchapo Fm. and remains high in the early Cambrian Xiaoyanxi Fm. (Fig. 2), suggesting siliceous sponge proliferation along the continental slope across the Ediacaran–Cambrian boundary. It should be noted that while the estimated amount of Si derived from sponges depends on both the prescribed seawater δ30Si value and on the sponge Si stable isotope fractionation (Fig. 2b), temporal trends in fsponge are unaffected by these estimates, provided they remained constant (Fig. 2c–f). Details of the mass balance are reported in Supplementary Note 2, and the estimated uncertainty on fsponge in Supplementary Data 6).
Diagenetic preservation of TOC and δ13Corg
TOC concentrations increase upsection from ~0.2 to 15.1 wt%, while δ13Corg increases from −35 to −29‰ up to 100 m and decreases higher up in the section to −33.1 to −33.5 δ13Corg (Fig. 1b). From the covariation of organic carbon concentrations with fsponge (Fig. 2c), we suggest that organic carbon transfer rates increased at the same time as siliceous sponges became more abundant. A more efficient biological pump would have led to higher organic carbon burial fluxes. The resulting anoxia would then have enhanced the organic carbon burial efficiency. The simultaneous increase of δ13Corg (Fig. 1a) supports enhanced organic carbon burial efficiency, as burial of isotopically light organic carbon increases the residual seawaters’ δ13C. The higher organic carbon burial efficiency led to increased δ13Corg, simultaneously across a range of slope-to-basin settings on the Yangtze Platform42. This observation is compatible with increased atmospheric oxygen concentrations that oxygenated seawater at short time scales < 1 kyr.
Our proxy data provide evidence that TOC and δ13Corg were not diagenetically modified. If significant post-depositional organic carbon oxidation had occurred, TOC concentrations would be underestimated and δ13Corg shifted (note that in this analysis we excluded samples that have low TOC concentrations in which even minor loss of isotopically fractionated C can be manifested in bulk rock C isotope ratios; see Supplementary Note 3 and Supplementary Fig. 6). However, the significant correlations between TOC and both the Ni and the Cu EFs, respectively (Fig. 3), suggest limited post-depositional organic carbon oxidation, as organic matter is less resistant to bacterial remineralization compared to Ni and Cu that are co-deposited at high organic matter fluxes and immobilized when reducing conditions are met43. If post-depositional organic carbon losses were indeed low, δ13C has not been substantially altered either.
TOC concentrations, EFs (Al and PAAS-normalized element concentrations) of redox-sensitive trace elements and CeN/CeN* at the Lijiatuo section. a Total organic carbon concentrations; b–i EFs of P, Ba, Cu, Ni, U, Mo, Cr and V are highest in organic-rich sediments and suboxic water as indicated by CeN/CeN* (j, horizontal gray shades indicate the most oxic conditions, i.e., CeN/CeN* < 0.6). Open symbols mark samples with PrN/YbN > 1, indicating flat, non-seawater REE patterns (cf. Fig. 1). Samples with <3% detritus are not shown due to potential normalization artifacts resulting from propogation of analytical uncertainties43
Seawater oxygenation during sponge expansion
The TOC and δ13C evidence for enhanced organic carbon burial in deep water depositional settings is corroborated by evidence for the corresponding decrease in the DOC pool size, and for the redox states in seawater and sediment.
Ge/Si(clay) is diagnostic of the DOC concentration in seawater20, 44. During initial sponge expansion, the Ge/Si ratios of clay minerals (estimated assuming Ge/Si of silica = 0.5 and using Al2O3 concentrations; Supplementary Fig. 2) continuously decreased (from ~50 to 10 µmol mol−1; Fig. 2e). This observation suggests that the DOC pool size decreased. The rationale for this interpretation is that Ge forms organic complexes through chelation with organic ligands45 that subsequently adsorb onto clay mineral surfaces. Therefore, clays obtain increasing Ge/Si with increasing DOC concentration in seawater. Bulk sediment Ge/Si ranges from 0.43 to 5.04 µmol mol−1 and increases with clay mineral abundances (Supplementary Fig. 2). The calculated Ge/Si ratios of illite, the predominant clay mineral in our Late Ediacaran samples (Supplementary Data 2, 7), are in the range of Lower Ediacaran (Doushantuo Fm.) chert nodules (~20–400 µmol mol−1), indicating DOC-rich seawater44. A decrease in Ge/Si during the latest Ediacaran to Lower Cambrian to Ge/Si < 20 µmol mol−1, similar to modern clay values (up to 24 µmol Ge per mol Si46) and similar to Ediacaran–Cambrian cherts (between 1 and 10 µmol Ge per mol Si20) suggests a decreasing DOC pool size.
A decreasing DOC pool size during the Precambrian–Cambrian transition is also supported by YN/HoN ratios that decrease from ~2.1 to 1.0 (Fig. 2f). Fractionation of Y from Ho in seawater is controlled by surface complexation onto inorganic metal oxide functional groups or organic substances47. The ligands for surface complexation are predominantly provided by organic particles48 and for most organic ligands Ho is preferentially complexed over Y49.
Contemporary seawater oxygenation is suggested by negative Ce anomalies (CeN/Ce*N < 1), i.e., a redox-controlled Ce-deficiency relative to Pr and Nd in bulk rock (Fig. 3c). Ce anomalies show decreasing values with increasing fsponge (Fig. 2d). The primary source of REEs, including Ce, is seawater from which REEs are scavenged by authigenic mineral formation at the sediment–water interface50 (Supplementary Note 4). CeN/Ce*N records the water redox state, as indicated by typical seawater REE patterns in bulk sediment (Supplementary Fig. 1). The CeN/Ce*N ratio is ~1 at the sections’ base and decreases to values as low as 0.42 at the top of the Liuchapo Fm. CeN/Ce*N in seawater decreases when soluble Ce(III) is oxidized to insoluble Ce(IV) relative to the adjacent REEs51. Decreasing CeN/Ce*N over the terminal Ediacaran documented here for the slope-to-basin setting at the Lijiatuo section has also been recorded in contemporaneous shallow-water carbonates of the Dengying Formation52. This trend suggests that this seawater oxygenation trend has at least a regional, if not a spatially even larger significance.
High EFs relative to the Post-Archean Australian Shale (PAAS)53 of redox-sensitive elements indicate reducing conditions. EFs are as high as 17 (Cu), 14 (Ni), 1218 (U), 433 (Mo), 40 (Cr) and 397 (V) (Fig. 3). Notably though, the highest EFs and thus most reducing sedimentary conditions are observed in samples with a substantial Ce-anomaly, i.e., where conditions in the seawater immediately above the sediment were oxidizing. This apparent conflict between redox-proxies is well known in Ediacaran to lower Cambrian cherts. It was suggested that a biogenic silica source derived from the oxic part of the water column was the carrier of low CeN/Ce*N to reducing sediments17. However, silica is not a major carrier of REE (Supplementary Fig. 7). Another possibility is that enhanced transfer of organic carbon from suboxic seawater to sediment reduced dissolved oxygen consumption in seawater. The higher oxygen levels led to oxidation of Fe and Mn and formation of Fe-Mn oxyhydroxides in the water column. V and Cr (and also other trace metals) were then attached to Fe-Mn oxyhydroxide surfaces and transferred to sediment. At the same time high TOC caused reducing conditions in the sediment where V and Cr, unlike Fe and Mn, were immobilized by complexation with humic and fulvic acids43. The VEF- CeN/Ce*N and the CrEF- CeN/Ce*N relationships (Fig. 3h, i) substantiate the link between organic matter burial, oxygen increase and coupling to the redox cycling of Mn and Fe43. High organic matter fluxes and enhanced Fe-Mn-oxyhydroxide shuttling of redox-sensitive metals in suboxic water to reducing sediments thus provides an explanation of the simultaneous enrichment of trace metals in sediments (VEF and CrEF) and oxygenation of bottom water (CeN/Ce*N), highlighting the redox contrast between seawater and sediment.
Such redox contrast between sediment and the overlying water column would lead to a redox gradient within shallow sediment. We argue that the high EFs of P (up to 80,000) and Ba (up to 300) (Fig. 3b, c) reflect their retention at the suboxic water–sediment interface. The simultaneous enrichment of Ba and P can be caused by both high productivity and by increasing oxygen levels in sediment54. Ba immobilization as barite occurs at the sulfate/sulfide redox interface55 and P accumulates under (sub-)oxic conditions through the bacterial formation of refractory P-compounds56. We argue that at the Lijiatuo section Ba- and P enrichment is primarily due to an oxygen increase in shallow sediment in contact with suboxic seawater. The reason is that Ba is present in form of diagenetic barite fronts that indicate Ba mobilization in underlying, reducing sediments, upward migration and precipitation at the sulfate/sulfide redox interface in shallow sediment. The correlation of P- and Ba enrichment thus suggests that P-immobilization is redox-controlled as well and occurred close to the water-sediment interface. The cumulative evidence for enhanced carbon burial efficiency, a decreased DOC pool, and higher dissolved oxygen concentrations during increased sponge spicule deposition suggests a causal relationship.
Filter feeding sponges as trigger for seawater oxygenation
A growing population of siliceous sponges on the Late Precambrian continental slope provides an explanation for the redox changes deduced from our geochemical proxy data. According to the hypothesis by Lenton et al.12 sponges have reduced the respiratory oxygen consumption in shallow seawater by removing DOC and fine POC (<10 μm57) from seawater by filter-feeding58. As a result, they shifted the oxygen demand by organic carbon oxidation to greater depth. This mechanism resulted in a net transfer of organic carbon to sediment and had large effects on seawater geochemistry13. Our data suggest that these effects included enhanced transfer of organic carbon from seawater to sediment, redox-sensitive trace metal enrichment in sediment, a decrease in the DOC pool size and increased dissolved oxygen levels. Seawater oxygenation might have been amplified by redox-controlled P immobilization and possibly by P-sequestration by sponge symbionts15, resulting in reduced dissolved P concentrations in seawater that limited primary productivity and oxygen demand by oxidation of organic carbon even further12.
The causality dilemma of oxygen and sponges
Increased oxygen levels would thus have been triggered by increased organic carbon extraction from seawater through filter feeding by sponges. However, it is unclear whether sponges expanded in oxygen-poor seawater and caused an increase in oxygen levels, or whether sponges rather expanded as consequence of seawater oxygen concentrations exceeding their minimum requirements.
If sponge expansion was impeded by a lack of oxygen in seawater, an initial increase in oxygen levels must have occurred, triggering their expansion. One possible trigger is a sea level fall in an ocean stratified with respect to dissolved O2 that would shift the redoxcline relative to the depositional site. This shift would cause a change in the sedimentary facies and in the organic carbon isotope composition. δ13Corg would be up to 2‰ lower through the decreasing contribution of organic carbon derived from chemoautotrophs59. We consider a falling sea level as cause for oxygen increase at Lijiatuo section unlikely, because positive shifts in δ13Corg have been observed in different paleo water depths on the Yangtze Platform42. Moreover, a regression-related positive δ13Corg shift conflicts with globally increasing δ13C records60 and a simultaneously rising global sea level61. On the Yangtze platform, this rising sea level is documented in transgressive system tracts in shallow water sections62.
We rather suggest that the observed facies change and increase in δ13Corg result from sponge expansion. Sponge expansion would increase the organic carbon burial efficiency, deplete seawater 12C, and raise the δ13C of subsequently produced organic matter. Some modern sponges can tolerate oxygen concentrations as low as 0.5–4% of present atmospheric levels, i.e., <11 µmol l−1 O2 for at least a portion of their life cycle24, giving further credibility to the potential role of sponges as triggers the Late Proterozoic oxygenation12, 63.
Potential effects of radiolarians and non-siliceous sponges
The silicon isotope record at Lijiatuo section is also compatible with deposition of radiolarians that carry 28Si-enriched silicon in their tests. Upon their death, radiolarians would have led to the rapid transfer of organic carbon to sediment. Therefore, radiolarians and sponges potentially affect the δ30Si and redox records alike and might have both contributed to seawater oxygenation. While the Si stable isotope fractionation by radiolarians is poorly constrained, it is known that modern radiolarians do not fractionate 28Si to the same extent as sponges64. A fractionation factor that is lower than that for sponge spicule formation implies that radiolarian tests are present in higher absolute abundance in chert (cf. Fig. 2b). This implication is not supported by the petrographic evidence. Radiolarian tests and sponge spicules might have both been deposited. We note, however, that the petrographic evidence for sponge spicules together with the good TOC- fsponge correlation (Fig. 2c) suggests a high fidelity of fsponge as a proxy for the siliceous sponge spicule abundance. While radiolarians might have contributed to enhanced organic carbon burial and seawater oxygenation, their presence is not unequivocally shown by fossil evidence (Supplementary Fig. 5), and therefore we suggest that sponges were the decisive ecosystem engineers.
We note that the proliferation of non-siliceous sponges, undetectable by Si stable isotopes, might have contributed to the observed changes in redox conditions too. Again, the overall good TOC- fsponge correlation suggests that sponges with siliceous spicules were the predominant drivers of enhanced carbon transfer to the benthos. Their predominance is likely in light of high Si levels in Precambrian seawater65, as even in modern, Si-depleted seawater ~75% of sponges build siliceous spicules57.
The changing biogeochemistry of marine ecosystems
With the appearance of filter-feeding benthic sponges, the biogeochemistry of the Late Neoproterozoic marine ecosystem changed fundamentally13. In Fig. 4, we summarize processes (a–h) that according to our model emerged as a result of sponge expansion and ensuing seawater oxygenation in the Late Precambrian.
Couplings in the Late Neoproterozoic to Cambrian marine ecosystem and their effects on the oxygen concentration in the water column. a Effects resulting from the expansion of sponges. Processes a–h are denoted by arrows and are detailed in the text. Arrows denote positive couplings (enhancing mechanisms) and circles negative couplings (inhibiting mechanisms). b With expansion of sponges the oxygen minimum zone shifted towards greater water depth, increasing the benthic oxygen inventory at the continental slope
Filtration of organic matter from seawater resulted in increased dissolved oxygen levels in shallow water (process a) through the shift in oxygen demand to depth (Fig. 4b). The selective removal of DOC and fine POC promoted the evolution of larger, eukaryotic primary producers, increasing organic carbon sinking rates and lowering re-oxidation rates in the upper water column12 (process b), contributing to the descend of the oxygen minimum zone (Fig. 4b). Enhanced organic carbon transfer to depth (process c) led to reducing conditions in sediment and subsequently to enhanced carbon burial that might have increased atmospheric oxygen concentrations. Diffusion of oxygen-rich seawater into sediments (process d) caused the establishment of a redox gradient close to the water-sediment interface. Under (sub-) oxic conditions, i.e., non-zero oxygen concentrations at the water-sediment interface, phosphorus was immobilized through the bacterial formation of refractory P-compounds56, (process e). Moreover, P-sequestration by sponge symbionts15 would have contributed to reduced levels of dissolved P (process f). As P is the limiting nutrient on geological time scales66, the reduced dissolved P inventory limited primary productivity, the sinking flux of organic matter and thus decreased the respiratory oxygen demand in seawater12 (process g). These couplings (between benthic oxygen concentration, dissolved P concentration, primary productivity and dissolved oxygen levels) created a positive feedback loop (green arrows) that led to a more oxygenated ocean. While full ocean-atmosphere oxygenation has extended until the emergence of land plants67, the seawater dissolved oxygen increase related to siliceous sponge expansion has potentially exceeded a necessary threshold for the evolution and distribution of animals with high oxygen demands63 (process h) and might thus have been critical for the Cambrian bioradiation. With the subsequent invention of bioturbation oxygen levels declined in Cambrian Stages 3 and 4 (~ 521 to ~ 509 Ma)68.
We have suggested that the appearance of sponges has ultimately raised seawater dissolved oxygen levels by reducing sedimentary P-recycling and primary productivity. Whether these processes also led to atmospheric oxygen increase depends on whether the O2 availability from enhanced organic carbon burial overcompensated the reduction in O2 production that resulted from reduced primary productivity caused by lowered P-levels in seawater.
Methods
Silicon stable isotope analysis
Silicon stable isotope ratios were determined on a Thermo Neptune multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) equipped with a Neptune Plus Jet Interface at GFZ Potsdam following protocols detailed in Oelze et al.69. Samples were diluted to 0.4–1 p.p.m. Si, doped with matching concentrations of Mg and introduced into an ESI APEX desolvator. Analyses were made in medium- or high resolution mode with typical signal intensities > 10 V on a Faraday cup (1011 Ω) for 28Si; blank intensities were usually < 5 mV. Instrumental mass bias correction was achieved by standard-sample bracketing after internal correction of mass bias drift by Mg isotope ratios. The 29Si/28Si and 30Si/28Si isotope ratios are reported as per mil deviation from the international reference material NIST 8546 aka. NBS 28, i.e., by multiplying Equation 1 with 103:
where x denotes 29 or 30. We abbreviate δ(x/28Si) NBS 28*103 as δxSi and report average δ-values obtained from between 4 and 7 replicate measurements of the same analyte solution and their 95% confidence interval (=t∙SD/√n), which indicates instrument repeatability. Quality control standards were regularly measured and yielded results in agreement with published data (Supplementary Data 8). Results from solution Si stable isotope analyses are compiled in Supplementary Data 4.
Organic carbon concentration and isotope analysis
The TOC concentration and δ13Corg were determined on decarbonated samples using an elemental analyzer NA1500 at GFZ Potsdam. Decarbonation was achieved by adding adequate volumes of 20% HCl to the sample powder in silver capsules and heating to dryness at 75 °C. Calibration was performed using an in-house urea standard and a certified standard (IAEA CH-7). The analytical precision for TOC concentrations was < 2%. The accuracy of carbon isotope analyses is estimated by repeat measurements of an internal soil reference sample (HEKATECH Boden3) that yielded accurate results, reproducible within 0.3‰ (1 SD).
Major- and trace element analysis
Major- and trace elements were determined using X-ray fluorescence analysis (XRF) and inductively coupled plasma mass spectrometry (ICP-MS). XRF analyses were carried out on powdered and fused samples using a Philips Panalytical PW2400 at the Institute of Applied Geosciences at the Technical University Berlin. The accuracy of XRF analyses was better than 5% RSD for most elements estimated using reference materials JR-1 and JR-2 (rhyolite). The uncertainty on trace element concentrations determined by ICP-MS (Actlabs, Canada) was estimated be < 10% based on analyses of reference materials DNC-1 (dolerite), W-2a (diabase), and BIR-1a (basalt). The uncertainty of REE + Y analyses is typically < 10% RSD, estimated from analyses of reference materials DNC-1, W-2a, BIR-1a and NCS DC70014 8 (ore).
We calculate Ce* for Ce-anomalies (CeN/Ce*N) according to Lawrence et al.70 (Equation 2). Using Nd and Pr to calculate Ce* precludes artificial anomalies that may arise from elevated La concentrations that result from the higher stability of La relative to other REE in solution. Ce-anomalies result from Ce(III) to Ce(IV) oxidation and enhanced scavenging onto reactive surfaces such as Fe-Mn-oxyhydroxides. As a result, Ce is becoming depleted in residual seawater and authigenic mineral phases precipitated thereof.
The subscript N denotes element concentrations normalized to post PAAS53.
We calculate the Ge/Si ratio of illite based on the illite stoichiometry, measured bulk rock Al2O3 concentrations and based on constant Ge/Si ratios of the pure silica end-member of 0.5 (Supplementary Fig. 2).
We report normalized element concentrations as element (El) enrichment factors (EF), defined as:
To preclude artefacts from normalization, EFs were calculated only for samples with > 3% detritus43. Moreover, we note that coefficients of variation are typically smaller for Al than for the redox-sensitive elements reported (Cu, Ni, U, V, Cr, P and Ba) except Mo.
Mineralogical composition
X-ray diffraction analyses (XRD) were carried out at the Institute of Applied Geosciences at the Technical University Berlin and GFZ Potsdam. Powdered samples were analyzed using an Iso-Debyeflex Philips PW 1050 diffractometer or a Panalytical Empyrean, respectively, both equipped with Cu-sources. XRD patterns were measured between 5–80° 2-theta and 2.5–80° 2-theta, respectively. Mineral phase composition and quantification were evaluated with the software X’Pert HighScore, and Autoquan. Uncertainties are estimated to < 10 wt% for major mineral constituents.
Siliceous sponge spicule abundance by mass balance
To quantify the abundance of Si derived from siliceous sponges we assume that the sampled cherts consist of four major Si sources. These Si sources are inorganic silica precipitated from seawater, Si in clay, Si in detrital quartz, and Si derived from sponge spicules. We formulate a Si stable isotope mass balance equation to calculate the abundance of sponge spicules in each sample:
where ‘detr’ and ‘auth’ denote detrital and authigenic clay, ‘qtz’ detrital quartz, ‘inorg’ seawater Si, and ‘sponge’ Si derived from siliceous sponge spicules. We derive the fractional contribution of clay (illite) from Al concentrations, validated by quantitative XRD analyses on selected samples (see Supplementary Data 7) and assume proportional detrital quartz abundances (see Supplementary Note 2). We assign isotope compositions and uncertainties to all compartments, and solve the equation for f(Si)sponge. We define the fraction of sponge-derived Si relative to the sum of Si derived from non-detrital components as fsponge. We assign the isotope composition of clay (detrital and authigenic) and detrital quartz the values of −0.8 ± 0.3‰ δ30Si and −0.3 ± 0.3‰ δ30Si, respectively. Seawater δ30Si is assumed to be 1.1 ± 0.5‰, the average of inorganic seawater silica precipitates from the Precambrian (avg.: 0.83‰ δ30Si; Ramseyer et al.21) and modern river water (avg.: 1.28‰ δ30Si; Frings et al.39). The sponge δ30Si is estimated to be −4.9‰ based on constant Si stable isotope fractionation by modern sponges at high Si concentrations modelled to be ε30Si = −6.02‰22. We assume that also the isotope composition of the inorganic seawater precipitate remained constant during the Precambrian–Cambrian transition within certain ranges. To account for possible species-dependent variations, we assume an uncertainty of 1‰ on the sponge δ30Si and 0.5‰ on seawater δ30Si. Resulting uncertainties on fsponge values were estimated using a Monte Carlo (MC) error propagation technique. For details about the mass balance approach see the Supplementary Note 2.
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information files.
References
Sperling, E. A. et al. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523, 3–6 (2015).
Chen, X. et al. Rise to modern levels of ocean oxygenation coincided with the Cambrian radiation of animals. Nat. Commun. 6, 7142 (2015).
Lee, C.-T. A. et al. Two-step rise of atmospheric oxygen linked to the growth of continents. Nat. Geosci. 9, 417–424 (2016).
Des Marais, D. J., Strauss, H., Summons, R. E. & Hayes, J. M. Carbon isotope evidence for the stepwise oxidation of the proterozoic environment. Nature 359, 605–609 (1992).
Canfield, D. E., Poulton, S. W. & Narbonne, G. M. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 315, 3–6 (2007).
Planavsky, N. J. et al. The evolution of the marine phosphate reservoir. Nature 467, 1088–1090 (2010).
Mills, B., Lenton, T. M. & Watson, A. J. Proterozoic oxygen rise linked to shifting balance between seafloor and terrestrial weathering. Proc. Natl Acad. Sci. USA 111, 9073–9078 (2014).
Lenton, T. M. & Watson, A. W. Biotic enhancement of weathering, atmospheric oxygen and carbon dioxide in the neoproterozoic. Geophys. Res. Lett. 31, 1–5 (2004).
Kump, L. R. Hypothesized link between Neoproterozoic greening of the land surface and the establishment of an oxygen-rich atmosphere. Proc. Natl Acad. Sci. USA 111, 14062–14065 (2014).
Logan, G. A., Hayes, J. M., Hieshima, G. B. & Summons, R. E. Terminal Proterzoic reorganization of biogeochemical cycles. Nature 376, 53–56 (1995).
Butterfield, N. J. Oxygen, animals and oceanic ventilation: an alternative view. Geobiology 7, 1–7 (2009).
Lenton, T. M., Boyle, R. A., Poulton, S. W., Shields-Zhou, G. A. & Butterfield, N. J. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat. Geosci. 7, 257–265 (2014).
Erwin, D. H. & Tweedt, S. Ecological drivers of the Ediacaran-Cambrian diversification of Metazoa. Evol. Ecol. 26, 417–433 (2012).
Dahl, T. W. et al. Reorganisation of Earth’s biogeochemical cycles briefly oxygenated the oceans 520 Myr ago. Geochem. Perspect. Lett. 3, 210–220 (2017).
Zhang, F. et al. Phosphorus sequestration in the form of polyphosphate by microbial symbionts in marine sponges. Proc. Natl Acad. Sci. USA 112, 4381–4386 (2015).
Sperling, E. A., Robinson, J. M., Pisani, D. & Peterson, K. J. Where’s the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology 8, 24–36 (2010).
Guo, Q. et al. Trace element chemostratigraphy of two Ediacaran–Cambrian successions in South China: implications for organosedimentary metal enrichment and silicification in the Early Cambrian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 254, 194–216 (2007).
Braun, A., Chen, J., Waloszek, D. & Maas, A. First Early Cambrian Radiolaria. Geol. Soc. Lond. Spec. Publ. 286, 143–149 (2007).
Fan, H., Wen, H., Zhu, X., Hu, R. & Tian, S. Hydrothermal activity during Ediacaran–Cambrian transition: silicon isotopic evidence. Precambrian Res. 224, 23–35 (2013).
Dong, L. et al. Germanium/silicon of the Ediacaran-Cambrian Laobao cherts: implications for the bedded chert formation and paleoenvironment interpretations. Geochem. Geophys. Geosyst. 16, 751–763 (2015).
Ramseyer, K. et al. Primary silica precipitate at the Precambrian/Cambrian boundary in the South Oman Salt Basin, Sultanate of Oman. Mar. Pet. Geol. 39, 187–197 (2013).
Wille, M. et al. Silicon isotopic fractionation in marine sponges: a new model for understanding silicon isotopic variations in sponges. Earth Planet. Sci. Lett. 292, 281–289 (2010).
Hendry, K. R., Georg, R. B., Rickaby, R. E. M., Robinson, L. F. & Halliday, A. N. Deep ocean nutrients during the last glacial maximum deduced from sponge silicon isotopic compositions. Earth. Planet. Sci. Lett. 292, 290–300 (2010).
Mills, D. B. et al. Oxygen requirements of the earliest animals. Proc. Natl Acad. Sci. USA 111, 4168–4172 (2014).
Shields-Zhou, G. A. & Zhu, M. Biogeochemical changes across the Ediacaran–Cambrian transition in South China. Precambrian Res. 225, 1–6 (2013).
Jiang, S.-Y., Pi, D.-H. & Schoene, B. Ages of the key boundaries during the Precambrian-Cambrian Interval in South China. In Geological Society of America Abstracts with Programs of GSA Denver Annual Meeting 42, 359 (2010).
Xu, L., Lehmann, B. & Mao, J. Seawater contribution to polymetallic Ni–Mo–PGE–Au mineralization in Early Cambrian black shales of South China: evidence from Mo isotope, PGE, trace element, and REE geochemistry. Ore Geol. Rev. 52, 66–84 (2013).
Guo, Q. et al. Carbon isotopic evolution of the terminal Neoproterozoic and early Cambrian: evidence from the Yangtze Platform, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 254, 140–157 (2007).
Tatzel, M., von Blanckenburg, F., Oelze, M., Schuessler, J. A. & Bohrmann, G. The silicon isotope record of early silica diagenesis. Earth Planet. Sci. Lett. 428, 293–303 (2015).
Siever, R. The silica cycle in the Precambrian. Geochim. Cosmochim. Acta 56, 3265–3272 (1992).
Kidder, D. L. & Erwin, D. H. Secular distribution of biogenic silica through the phanerozoic: comparison of silica-replaced fossils and bedded cherts at the series level. J. Geol. 109, 509–522 (2001).
Tarhan, L. G., Hood, A. V. S., Droser, M. L., Gehling, J. G. & Briggs, D. E. G. Exceptional preservation of soft-bodied Ediacara Biota promoted by silica-rich oceans. Geology 44, 951–954 (2016).
Yee, N., Phoenix, V. R., Konhauser, K. O., Benning, L. G. & Ferris, F. G. The effect of cyanobacteria on silica precipitation at neutral pH: implications for bacterial silicification in geothermal hot springs. Chem. Geol. 199, 83–90 (2003).
Geilert, S., Vroon, P. Z. & van Bergen, M. J. Effect of diagenetic phase transformation on the silicon isotope composition of opaline sinter deposits of Geysir, Iceland. Chem. Geol. 433, 57–67 (2016).
Isaacs, C. M. Influence of rock composition on kinetics of silica phase changes in the Monterey Formation, Santa Barbara area, California. Geology 10, 304 (1982).
Marin-Carbonne, J., Chaussidon, M. & Robert, F. Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts: implications for paleo-temperature reconstructions. Geochim. Cosmochim. Acta 92, 129–147 (2012).
Tréguer, P. J. et al. The silica balance in the world ocean: a reestimate. Science 268, 375–379 (1995).
De La Rocha, C. L., Brzezinski, M. A. & DeNiro, M. J. A first look at the distribution of the stable isotopes of silicon in natural waters. Geochim. Cosmochim. Acta. 64, 2467–2477 (2000).
Frings, P. J., Clymans, W., Fontorbe, G., De La Rocha, C. L. & Conley, D. J. The continental Si cycle and its impact on the ocean Si isotope budget. Chem. Geol. 425, 12–36 (2016).
Li, C. W., Chen, J. Y. & Hua, T. E. Precambrian sponges with cellular structures. Science 279, 879–882 (1998).
Mortlock, R. A. & Froelich, P. N. Hydrothermal germanium over the southern East pacific rise. Science 231, 43–45 (1986).
Guo, Q. et al. High resolution organic carbon isotope stratigraphy from a slope to basinal setting on the Yangtze Platform, South China: implications for the Ediacaran-Cambrian transition. Precambrian Res. 225, 209–217 (2013).
Tribovillard, N., Algeo, T. J., Lyons, T. W. & Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: an update. Chem. Geol. 232, 12–32 (2006).
Shen, B., Lee, C.-T. A. & Xiao, S. Germanium/silica ratios in diagenetic chert nodules from the Ediacaran Doushantuo formation, South China. Chem. Geol. 280, 323–335 (2011).
Pokrovsky, G. S. & Schott, J. Experimental study of the complexation of silicon and germanium with aqueous organic species: implications for germanium and silicon transport and Ge/Si ratio in natural waters. Geochim. Cosmochim. Acta. 62, 3413–3428 (1998).
Kurtz, A. C., Derry, L. A. & Chadwick, O. A. Germanium – silicon fractionation in the weathering environment. Geochim. Cosmochim. Acta. 66, 1525–1537 (2002).
Bau, M., Koschinsky, A., Dulski, P. & Hein, J. R. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Cosmochim. Acta. 60, 1709–1725 (1996).
Balistrieri, L., Brewer, P. G. & Murray, J. W. Scavenging residence times of trace metals and surface chemistry of sinking particles in the deep ocean. Deep Sea Res. A Oceanogr. Res. Pap. 28, 101–121 (1981).
Byrne, R. H. & Lee, J. H. Comparitive yttrium and rare earth element chemistries in sewater. Mar. Chem. 44, 121–130 (1993).
Murray, R. W., Buchholtz ten Brink, M. R., Gerlach, D. C., Price Russ, G. III & Jones, D. L. Rare earth, major, and trace elements in chert from the franciscan complex and monterey group, California: assessing REE sources to fine-grained marine sediments. Geochim. Cosmochim. Acta. 55, 1875–1895 (1991).
Bau, M. & Koschinsky, A. Oxidative scavenging of cerium on hydrous Fe oxide: evidence from the distribution of rare earth elements and yttrium between Fe oxides and Mn oxides in hydrogenetic ferromanganese crusts. Geochem. J. 43, 37–47 (2009).
Ling, H.-F. et al. Cerium anomaly variations in Ediacaran–earliest Cambrian carbonates from the Yangtze Gorges area, South China: implications for oxygenation of coeval shallow seawater. Precambrian. Res. 225, 110–127 (2013).
McLennan, S. M. Rare earth elements in sedimentary rocks; influence of provenance and sedimentary processes. Rev. Mineral. Geochem. 21, 169–200 (1989).
Schoepfer, S. D. et al. Total organic carbon, organic phosphorus, and biogenic barium fluxes as proxies for paleomarine productivity. Earth Sci. Rev. 149, 23–52 (2014).
Torres, M. E., Brumsack, H. J., Bohrmann, G. & Emeis, K. C. Barite fronts in continental margin sediments: a new look at barium remobilization in the zone of sulfate reduction and formation of heavy barites in diagenetic fronts. Chem. Geol. 127, 125–139 (1996).
Ingall, E. D., Bustin, R. M. & van Cappellen, P. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim. Cosmochim. Acta 57, 303–316 (1993).
Maldonado, M., Ribes, M. & van Duyl, F. C. in Advances in Marine Biology Vol. 62 (eds Becerro M. A., Uriz, M. J., Maldonado, M. & Turon, X.) Ch. 3 (Elsevier Ltd., 2012).
de Goeij, J. M., van den Berg, H., van Oostveen, M. M., Epping, E. H. G. & van Duyl, F. C. Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar. Ecol. Prog. Ser. 357, 139–151 (2008).
Ader, M. et al. A multilayered water column in the Ediacaran Yangtze platform? Insights from carbonate and organic matter paired δ13C. Earth Planet. Sci. Lett. 288, 213–227 (2009).
Maloof, A. C. et al. The earliest Cambrian record of animals and ocean geochemical change. Bull. Geol. Soc. Am. 122, 1731–1774 (2010).
Haq, B. U. & Schutter, S. R. A chronology of Paleozoic sea-level changes. Science 322, 64–68 (2008).
Li, D. et al. Carbon and strontium isotope evolution of seawater across the Ediacaran-Cambrian transition: evidence from the Xiaotan section, NE Yunnan, South China. Precambrian Res. 225, 128–147 (2013).
Mills, D. B. & Canfield, D. E. Oxygen and animal evolution: did a rise of atmospheric oxygen trigger the origin of animals? BioEssays 36, 1145–1155 (2014).
Fontorbe, G., Frings, P. J., De La Rocha, C. L., Hendry, K. R. & Conley, D. J. A silicon depleted North Atlantic since the Palaeogene: evidence from sponge and radiolarian silicon isotopes. Earth. Planet. Sci. Lett. 453, 67–77 (2016).
Racki, G. & Cordey, F. Radiolarian palaeoecology and radiolarites: is the present the key to the past? Earth Sci. Rev. 52, 83–120 (2000).
Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400, 525–531 (1999).
Lenton, T. M. et al. Earliest land plants created modern levels of atmospheric oxygen. Proc. Natl Acad. Sci. USA 113, 9704–9709 (2016).
Boyle, R. A. et al. Stabilization of the coupled oxygen and phosphorus cycles by the evolution of bioturbation. Nat. Geosci. 7, 671–676 (2014).
Oelze, M., Schuessler, J. & von Blanckenburg, F. Mass bias stabilization by Mg doping for Si stable isotope analysis by MC-ICP-MS. J. Anal. Atom. Spectrom. 31, 2094–2100 (2016).
Lawrence, M. G., Greig, A., Collerson, K. D. & Kamber, B. S. Rare earth element and yttrium variability in South East Queensland Waterways. Aquat. Geochem. 12, 39–72 (2006).
Acknowledgements
We are grateful for funding provided by the DFG through research group FOR736 ‘The Precambrian–Cambrian Ecosphere (R)evolution: Insights from Chinese microcontinents’ as well as for field guidance by S.-Y. Jiang, H.-F. Ling and others, and financing of field work through the NSFC. We thank B. Plessen for supporting C-isotope measurements, J.A. Schuessler for laboratory support, R. Naumann and C. Kotre for XRD analyses. Discussions with P.J. Frings, S.V. Hohl, B. Kamber, and J. Payne are appreciated.
Author information
Authors and Affiliations
Contributions
The research was planned by F.v.B. and D.H. M.T. collected samples and carried out Si isotope analyses. J.B. and M.T. calculated the mass balance. The data were interpreted by M.T. with input from F.v.B. and M.O. M.T. wrote the paper with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tatzel, M., von Blanckenburg, F., Oelze, M. et al. Late Neoproterozoic seawater oxygenation by siliceous sponges. Nat Commun 8, 621 (2017). https://doi.org/10.1038/s41467-017-00586-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-017-00586-5
This article is cited by
-
Hydroclimatic variations in the Tianshan Mountains based on grain size and geochemistry of core sediments since ∼1490 CE
Journal of Mountain Science (2023)
-
Quartz types, genesis and their geological significance within the Wufeng-Longmaxi Formation in north-western Hunan, China
Frontiers of Earth Science (2023)
-
Early Cambrian highly metalliferous black shale in South China: Cu and Zn isotopes and a short review of other non-traditional stable isotopes
Mineralium Deposita (2022)
-
Isotopic evidence for temperate oceans during the Cambrian Explosion
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.