Abstract
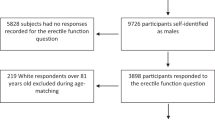
To determine the relationship between popular diets and erectile function we queried the National Health and Nutrition Examination Survey, a cross-sectional dataset, between 2001 and 2004. All men aged 18–85 who answered the prostate and dietary questionnaires were included. Diets were categorized as Mediterranean, low-fat, low-carbohydrate, or nonrestrictive. Multivariable models were created to determine the relationship between erectile function and each diet. Among 4027 men, 649 (16.1%) met criteria for a low-fat diet, 1085 (26.9%) for a Mediterranean diet, and 0 (0%) for a low-carbohydrate diet. 1999 men (49.6%) had some degree of erectile dysfunction. Men with nonrestrictive diets were more likely to endorse normal erectile function compared with those adhering to the Mediterranean or low-fat diets (both p < 0.05) on univariable analysis. Multivariable analysis controlling for age, comorbidities, activity level, and body mass index showed no differences in erectile function among men adhering to a low-fat, Mediterranean diet, or nonrestrictive diet. There was no association between specific diets and erectile function. While additional prospective research is required to corroborate these findings, these data support the notion that individualized diets should be tailored toward goals of weight loss and reduction of comorbidity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED. et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7:1598–607.
McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000;12 Suppl 4:S6–11.
Latini DM, Penson DF, Wallace KL, Lubeck DP, Lue TF. Clinical and psychosocial characteristics of men with erectile dysfunction: baseline data from ExCEED. J Sex Med. 2006;3:1059–67.
Lee JH, Ngengwe R, Jones P, Tang F, O’Keefe JH. Erectile dysfunction as a coronary artery disease risk equivalent. J Nucl Cardiol. 2008;15:800–3.
Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–41.
Martin-Morales A, Sanchez-Cruz JJ, Saenz de Tejada I, Rodriguez-Vela L, Jimenez-Cruz JF, Burgos-Rodriguez R. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569–74. discussion 574-575
Kovac JR, Labbate C, Ramasamy R, Tang D, Lipshultz LI. Effects of cigarette smoking on erectile dysfunction. Andrologia. 2015;47:1087–92.
Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–84.
Esposito K, Ciotola M, Giugliano F, De Sio M, Giugliano G, D’armiento M, et al. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int J Impot Res. 2006;18:405–10.
Khoo J, Piantadosi C, Worthley S, Wittert GA. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int J Obes. 2010;34:1396–403.
Kałka D, Domagała Z, Dworak J, Womperski K, Rusiecki L, Marciniak W, et al. Association between physical exercise and quality of erection in men with ischaemic heart disease and erectile dysfunction subjected to physical training. Kardiol Pol. 2013;71:573–80.
Searing L. The Big Number: 45 million Americans go on a diet each year. The Washington Post [Internet]. 2018 Jan 1[cited 2020 May 26]. Available from: https://www.washingtonpost.com/national/health-science/the-big-number-45-million-americans-go-on-a-diet-each-year/2017/12/29/04089aec-ebdd-11e7-b698-91d4e35920a3_story.html.
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41.
Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Stroke. 2000;31:2751–66.
WILLETT W EAT, DRINK, AND BE HEALTHY: the harvard medical school guide to healthy eating. Place of publication not identified: FREE Press; New York, NY; 2017.
Atkins RC. Dr. Atkins’ new diet revolution. 1st Avon pbk. ed. New York: Avon Books; 2002. 540 p.
Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7:121–34.
Dwyer J, Picciano MF, Raiten DJ, Members of the Steering Committee. Estimation of Usual Intakes: What We Eat in America–NHANES. J Nutr. 2003;133:609S–23S.
Raper N, Perloff B, Ingwersen L, Steinfeldt L, Anand J. An overview of USDA’s dietary intake data system. J Food Composition Anal. 2004;17:545–55.
He Z, Charness N, Bian J, Hogan WR Assessing the comorbidity gap between clinical studies and prevalence in elderly patient populations. In: 2016 IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI) [Internet]. Las Vegas, NV, USA: IEEE; 2016 [cited 7 Dec 2018]. p. 136–9. http://ieeexplore.ieee.org/document/7455853/.
NHANES 2001–2002 Laboratory Methods [Internet]. CDC, National Center for Health Statistics; [cited 20 Mar 2019]. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2001.
NHANES 2003–2004 Laboratory Methods [Internet]. CDC, National Center for Health Statistics; [cited 20 Mar 2019]. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2001.
Pirkle J. Laboratory Procedure Manual: Total Testosterone [Internet]. CDC; 2011-2012 [cited 2020 May 26]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/tst_g_met.pdf.
Mirel LB, Mohadjer LK, Dohrmann SM, Clark J, Burt VL, Johnson CL, et al. National Health and Nutrition Examination Survey: estimation procedures, 2007–2010. Vital Health Stat. 2013;2:1–17.
La J, Roberts NH, Yafi FA. Diet and Men’s Sexual Health. Sex Med Rev.2018;6:54–68.
Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano Iii INC, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med. 2010;7:156–65.
Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–75.
Maiorino MI, Bellastella G, Caputo M, Castaldo F, Improta MR, Giugliano D, et al. Effects of Mediterranean diet on sexual function in people with newly diagnosed type 2 diabetes: The MÈDITA trial. J Diabetes Complicat. 2016;30:1519–24.
Moran LJ, Brinkworth GD, Martin S, et al. Long-term effects of a randomised controlled trial comparing high protein or high carbohydrate weight loss diets on testosterone, SHBG, erectile and urinary function in overweight and obese men. Shore N, editor. PLoS One. 2016;11:e0161297.
de Souza ILL, Barros BC, de Oliveira GA, Queiroga FR, Toscano LT, Silva AS, et al. Hypercaloric diet establishes erectile dysfunction in rat: mechanisms underlying the endothelial damage. Front Physiol. 2017;8:760.
Alves-Pereira JL, Frantz EDC, Pires LAS, Babinski MA, da Fonte, Ramos C. Effects of a high energy density diet in the “corpus cavernosum” of mice. Int J Impot Res. 2019;31:126–31.
Yeşilli Ç, Yaman Ö, Anafarta K. Effect of experimental hypercholesterolemia on cavernosal structures. Urology. 2001;57:1184–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fantus, R.J., Halpern, J.A., Chang, C. et al. The association of popular diets and erectile function among men in the United States. Int J Impot Res 33, 548–555 (2021). https://doi.org/10.1038/s41443-020-0313-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-020-0313-x