Abstract
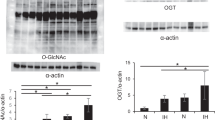
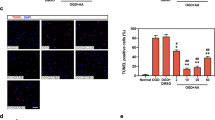
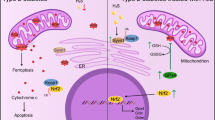
Previously, we showed that augmented O-linked N-acetylglucosaminylation (O-GlcNAcylation) mitigates cardiac remodeling in O-GlcNAc transferase-transgenic (Ogt-Tg) mice exposed to acute (2-week) intermittent hypoxia (IH) by suppressing nuclear factor of activated T cells (NFAT) and nuclear factor kappa B (NF-κB) via the O-GlcNAcylation of glycogen synthase kinase 3 beta (GSK-3β) and NF-κB p65. Because this effect is time dependent, we exposed Ogt-Tg mice to IH for 4 weeks (IH4W) in the present study. O-GlcNAcylation was significantly enhanced in Ogt-Tg mice vs. wild-type (WT) mice exposed to normoxia and IH4W. Total O-GlcNAcylation levels were significantly increased in WT and Ogt-Tg mice after IH4W vs. normoxia. After IH4W, Ogt-Tg mice displayed significantly exacerbated signs of cardiac hypertrophy and fibrosis in the right ventricles (RVs) but not the left ventricles (LVs). Echocardiography revealed IH4W-induced right ventricular dysfunction. Phosphorylated GSK-3β levels were increased in Ogt-Tg mice vs. WT mice after IH4W, whereas phosphorylated NF-κB p65 levels were unaffected. Mitophagy, which is associated with cardiac dysfunction, was increased in the RVs of Ogt-Tg mice after IH4W. Furthermore, the levels of phosphorylated dynamin-related protein 1 (p-Drp1) were significantly increased, and the expression of mitofusin-2 (MFN2) was significantly decreased. In human embryonic kidney cells, mitochondrial uncoupler-induced mitochondrial dysfunction was accelerated in Ogt-overexpressing cells. In addition to increasing the levels of phosphorylated Smad2, IH4W promoted cardiac fibrosis in the RVs of Ogt-Tg mice. Thus, augmented O-GlcNAcylation may aggravate IH4W-induced right ventricular dysfunction and remodeling by promoting hypertrophy, mitophagy, and fibrosis via GSK-3β inactivation, an increased p-Drp-1/MFN2 ratio, and Smad2 activation, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987;104:1157–64.
Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007;446:1017–22.
Iyer SP, Hart GW. Dynamic nuclear and cytoplasmic glycosylation: enzymes of O-GlcNAc cycling. Biochemistry. 2003;42:2493–9.
Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–82.
Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, et al. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol. 2009;296:H13–28.
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58.
Chen LM, Kuo WW, Yang JJ, Wang SG, Yeh YL, Tsai FJ, et al. Eccentric cardiac hypertrophy was induced by long-term intermittent hypoxia in rats. Exp Physiol. 2007;92:409–16.
Pena E, Siques P, Brito J, Arribas SM, Boger R, Hannemann J, et al. Nox2 upregulation and p38alpha MAPK activation in right ventricular hypertrophy of rats exposed to long-term chronic intermittent hypobaric hypoxia. Int J Mol Sci. 2020;21:8576.
Guan P, Sun ZM, Wang N, Zhou J, Luo LF, Zhao YS, et al. Resveratrol prevents chronic intermittent hypoxia-induced cardiac hypertrophy by targeting the PI3K/AKT/mTOR pathway. Life Sci. 2019;233:116748.
Xie S, Deng Y, Pan YY, Ren J, Jin M, Wang Y, et al. Chronic intermittent hypoxia induces cardiac hypertrophy by impairing autophagy through the adenosine 5’-monophosphate-activated protein kinase pathway. Arch Biochem Biophys. 2016;606:41–52.
Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol. 2008;294:C1509–20.
Genovese A, Chiariello M, Cacciapuoti AA, De Alfieri W, Latte S, Condorelli M. Inhibition of hypoxia-induced cardiac hypertrophy by verapamil in rats. Basic Res Cardiol. 1980;75:757–63.
Jensen RV, Zachara NE, Nielsen PH, Kimose HH, Kristiansen SB, Botker HE. Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc Res. 2013;97:369–78.
Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007;292:C178–87.
Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol. 2007;293:H1391–9.
Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–12.
Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics. 2012;44:162–72.
Wang D, Hu X, Lee SH, Chen F, Jiang K, Tu Z, et al. Diabetes exacerbates myocardial ischemia/reperfusion injury by down-regulation of microRNA and up-regulation of O-GlcNAcylation. JACC Basic Transl Sci. 2018;3:350–62.
Nakagawa T, Furukawa Y, Hayashi T, Nomura A, Yokoe S, Moriwaki K, et al. Augmented O-GlcNAcylation attenuates intermittent hypoxia-induced cardiac remodeling through the suppression of NFAT and NF-kappaB activities in mice. Hypertens Res. 2019;42:1858–71.
Moriwaki K, Asahi M. Augmented TME O-GlcNAcylation promotes tumor proliferation through the inhibition of p38 MAPK. Mol Cancer Res. 2017;15:1287–98.
Nishioka S, Yoshioka T, Nomura A, Kato R, Miyamura M, Okada Y, et al. Celiprolol reduces oxidative stress and attenuates left ventricular remodeling induced by hypoxic stress in mice. Hypertens Res. 2013;36:934–9.
Kato R, Nomura A, Sakamoto A, Yasuda Y, Amatani K, Nagai S, et al. Hydrogen gas attenuates embryonic gene expression and prevents left ventricular remodeling induced by intermittent hypoxia in cardiomyopathic hamsters. Am J Physiol Heart Circ Physiol. 2014;307:H1626–33.
Yamashita C, Hayashi T, Mori T, Tazawa N, Kwak CJ, Nakano D, et al. Angiotensin II receptor blocker reduces oxidative stress and attenuates hypoxia-induced left ventricular remodeling in apolipoprotein E-knockout mice. Hypertens Res. 2007;30:1219–30.
Hayashi T, James TN, Buckingham DC. Ultrastructure and immunohistochemistry of the coronary chemoreceptor in human and canine hearts. Am Heart J. 1995;129:946–59.
Ogihara Y, Yamada N, Dohi K, Matsuda A, Tsuji A, Ota S, et al. Utility of right ventricular Tei-index for assessing disease severity and determining response to treatment in patients with pulmonary arterial hypertension. J Cardiol. 2014;63:149–53.
Sun LY, Zhao H, Kang Y, Shen XD, Cai ZY, Shen JY, et al. Two-dimensional echocardiography in the assessment of long-term prognosis in patients with pulmonary arterial hypertension. PLoS One. 2014;9:e114443.
Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107:727–32.
Tugcu A, Guzel D, Yildirimturk O, Aytekin S. Evaluation of right ventricular systolic and diastolic function in patients with newly diagnosed obstructive sleep apnea syndrome without hypertension. Cardiology. 2009;113:184–92.
Tugcu A, Yildirimturk O, Tayyareci Y, Demiroglu C, Aytekin S. Evaluation of subclinical right ventricular dysfunction in obstructive sleep apnea patients using velocity vector imaging. Circ J. 2010;74:312–9.
Cho HJ, Heo W, Han JW, Lee YH, Park JM, Kang MJ, et al. Chronological change of right ventricle by chronic intermittent hypoxia in mice. Sleep. 2017;40.
Smith KA, Waypa GB, Dudley VJ, Budinger GRS, Abdala-Valencia H, Bartom E, et al. Role of hypoxia-inducible factors in regulating right ventricular function and remodeling during chronic hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 2020;63:652–64.
Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93.
Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–12.
Gurel Z, Sieg KM, Shallow KD, Sorenson CM, Sheibani N. Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol Vis. 2013;19:1047–59.
Park MJ, Kim DI, Lim SK, Choi JH, Han HJ, Yoon KC, et al. High glucose-induced O-GlcNAcylated carbohydrate response element-binding protein (ChREBP) mediates mesangial cell lipogenesis and fibrosis: the possible role in the development of diabetic nephropathy. J Biol Chem. 2014;289:13519–30.
Yokoe S, Asahi M, Takeda T, Otsu K, Taniguchi N, Miyoshi E, et al. Inhibition of phospholamban phosphorylation by O-GlcNAcylation: implications for diabetic cardiomyopathy. Glycobiology. 2010;20:1217–26.
Nomura A, Yokoe S, Tomoda K, Nakagawa T, Martin-Romero FJ, Asahi M. Fluctuation in O-GlcNAcylation inactivates STIM1 to reduce store-operated calcium ion entry via down-regulation of Ser(621) phosphorylation. J Biol Chem. 2020;295:17071–82.
Pennanen C, Parra V, Lopez-Crisosto C, Morales PE, Del Campo A, Gutierrez T, et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci. 2014;127:2659–71.
Vasquez-Trincado C, Garcia-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, et al. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594:509–25.
Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–83.
Saadat S, Noureddini M, Mahjoubin-Tehran M, Nazemi S, Shojaie L, Aschner M, et al. Pivotal role of TGF-beta/Smad signaling in cardiac fibrosis: non-coding RNAs as effectual players. Front Cardiovasc Med. 2020;7:588347.
Acknowledgements
We are grateful to Yudai Yamaguchi, Yuhei Yamanaka, Maki Tamura (research assistants at the Department of Cardiovascular Pharmacotherapy and Toxicology, Faculty of Pharmacy) and Yumiko Okumura (Osaka Medical and Pharmaceutical University) for their expert technical assistance.
Funding
This study was partially supported by Grant-in-Aid for Scientific Research (C) no. 25460397 from the Japan Society for the Promotion of Science (MA) under the Ministry of Education, Science, Culture, Sports and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yokoe, S., Hayashi, T., Nakagawa, T. et al. Augmented O-GlcNAcylation exacerbates right ventricular dysfunction and remodeling via enhancement of hypertrophy, mitophagy, and fibrosis in mice exposed to long-term intermittent hypoxia. Hypertens Res 46, 667–678 (2023). https://doi.org/10.1038/s41440-022-01088-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01088-8
Keywords
This article is cited by
-
Could the control of O-GlcNAcylation play a key role in cardiac remodeling?
Hypertension Research (2023)
-
Stomatin-like protein 2 deficiency exacerbates adverse cardiac remodeling
Cell Death Discovery (2023)