Abstract
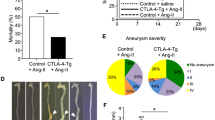
A subset of interleukin (IL)-17A-producing γδ T cells called γδT17 cells may contribute to progression of hypertension. γδT17 cell development is in part dependent upon IL-23 receptor (IL-23R) stimulation. We hypothesized that angiotensin (Ang) II-induced blood pressure (BP) elevation and vascular injury would be blunted in Il23r knock-in (Il23rgfp/gfp) mice deficient in functional IL-23R. To test this hypothesis, we infused wild-type (WT) and Il23rgfp/gfp mice with Ang II (490 ng/kg/min, SC) for 7 or 14 days. We recorded BP by telemetry, assessed vascular function and remodeling using pressurized myography, and profiled T cell populations and cytokine production by flow cytometry. An additional set of Il23rgfp/gfp mice was infused with Ang II for 7 days and injected with interferon (IFN)-γ-neutralizing or control antibodies. Il23rgfp/gfp mice had smaller and stiffer mesenteric arteries and were not protected against Ang II-induced BP elevation. BP was higher in Il23rgfp/gfp mice than WT mice from day 3 until day 9 of Ang II infusion. Il23rgfp/gfp mice had less γδT17 cells and more IFN-γ-producing γδ, CD4+, and CD8+ T cells than WT mice. Seven days of Ang II infusion led to increased IFN-γ-producing γδ, CD4+, and CD8+ T cells in Il23rgfp/gfp mice, whereas only IFN-γ-producing γδ T cells were increased in WT mice. Blocking IFN-γ with a neutralizing antibody reduced the pressor response to 7 days of Ang II infusion in Il23rgfp/gfp mice. Functional IL-23R deficiency was associated with increased IFN-γ-producing T cells and exaggerated initial development of Ang II-induced hypertension, which was in part mediated by IFN-γ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017;317:165–82.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–60.
Zhou B, Danaei G, Stevens GA, Bixby H, Taddei C, Carrillo-Larco RM, et al. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. 2019;394:639–51.
Chobanian AV. Impact of nonadherence to antihypertensive therapy. Circulation 2009;120:1558–60.
Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharm. 2019;176:1818–28.
Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, et al. gammadelta T Cells Mediate Angiotensin II-Induced Hypertension and Vascular Injury. Circulation 2017;135:2155–62.
Higaki A, Caillon A, Paradis P, Schiffrin EL. Innate and innate-like immune system in hypertension and vascular injury. Curr Hypertens Rep. 2019;21:4.
Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA. 2009;106:2307–12.
Kohlgruber AC, Gal-Oz ST, Lamarche NM, Shimazaki M, Duquette D, Koay H-F, et al. T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol. 2018;19:464–74.
Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010;55:500–7.
Karbach S, Croxford AL, Oelze M, Schüler R, Minwegen D, Wegner J, et al. Interleukin 17 Drives Vascular Inflammation, Endothelial Dysfunction, and Arterial Hypertension in Psoriasis-Like Skin Disease. Arteriosclerosis, Thrombosis, Vasc Biol. 2014;34:2658–68.
Yao W, Sun Y, Wang X, Niu K. Elevated serum level of interleukin 17 in a population with prehypertension. J Clin Hypertens (Greenwich). 2015;17:770–4.
Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100.
Muschaweckh A, Petermann F, Korn T. IL-1beta and IL-23 Promote Extrathymic Commitment of CD27(+)CD122(−) gammadelta T Cells to gammadeltaT17 Cells. J Immunol. 2017;199:2668–79.
Schaalan M, Mohamed W, Rahmo R. Association of cardiac NT pro-beta-type natriuretic peptide with metabolic and endothelial risk factors in young obese hypertensive patients: a perspective on the hypothalamic pituitary adrenal axis activation. Diabetol Metab Syndr. 2016;8:52.
Ye J, Wang Y, Wang Z, Liu L, Yang Z, Wang M, et al. The Expression of IL-12 family members in patients with hypertension and its association with the occurrence of carotid atherosclerosis. Mediators Inflamm 2020;2020:e2020.
Zhang M, Cai ZR, Zhang B, Cai X, Li W, Guo Z, et al. Functional polymorphisms in interleukin-23 receptor and susceptibility to coronary artery disease. DNA Cell Biol. 2014;33:891–7.
Lee E, Kim N, Kang J, Yoon S, Lee HA, Jung H, et al. Activated pathogenic Th17 lymphocytes induce hypertension following high-fructose intake in Dahl salt-sensitive but not Dahl salt-resistant rats. Dis Model Mech. 2020;13:dmm044107.
Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–8.
Schiffrin EL. How structure, mechanics, and function of the vasculature contribute to blood pressure elevation in hypertension. Can J Cardiol. 2020;36:648–58.
Saleh MA, Norlander AE, Madhur MS. Inhibition of interleukin 17-A but not Interleukin-17F signaling lowers blood pressure and reduces end-organ inflammation in angiotensin II-induced hypertension. JACC Basic Transl Sci. 2016;1:606–16.
Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A-/- mice. Hypertension 2015;65:569–76.
Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, et al. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin ii-induced hypertension. Hypertension 2014;63:565–71.
Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, et al. Interferon-gamma signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 2012;60:1430–6.
Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood 2013;121:2402–14.
Lückel C, Picard FSR, Huber M. Tc17 biology and function: Novel concepts. Eur J Immunol. 2020;50:1257–67.
Fiala GJ, Gomes AQ, Silva-Santos B. From thymus to periphery: Molecular basis of effector gammadelta-T cell differentiation. Immunol Rev. 2020;298:47–60.
Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods. 2004;293:127–42.
Cibrián D, Sánchez-Madrid F. CD69: From activation marker to metabolic gatekeeper. Eur J Immunol. 2017;47:946–53.
Acknowledgements
We are grateful to Veronique Michaud for excellent technical support.
Funding
The work of the authors was supported by the Canadian Institutes of Health Research (CIHR) First Pilot Foundation Grant 143348, a Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, a Distinguished James McGill Professorship Award, and by the Canada Fund for Innovation, to ELS, by the Fonds de recherche Santé Quebec (FRQS) bourse 289184, Lady Davis Institute/TD Bank Studentship award and CIHR Canada Graduate Scholarship to BGS and by the McGill Department of Medicine Gordon Phillips Fellowship to KC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shokoples, B.G., Comeau, K., Higaki, A. et al. Angiotensin II-induced a steeper blood pressure elevation in IL-23 receptor-deficient mice: Role of interferon-γ-producing T cells. Hypertens Res 46, 40–49 (2023). https://doi.org/10.1038/s41440-022-01055-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01055-3
Keywords
This article is cited by
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
Winners for the 14th Hypertension Research Awards and outstanding papers in Hypertension Research
Hypertension Research (2024)
-
The renin-angiotensin-aldosterone system: a new look at an old system
Hypertension Research (2023)
-
A new face among our Associate Editors
Hypertension Research (2023)