Abstract
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, the possible roles of renin–angiotensin system (RAS) inhibitors in COVID-19 have been debated as favorable, harmful, or neutral. Angiotensin-converting enzyme 2 (ACE2) not only is the entry route of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection but also triggers a major mechanism of COVID-19 aggravation by promoting tissue RAS dysregulation, which induces a hyperinflammatory state in several organs, leading to lung injury, hematological alterations, and immunological dysregulation. ACE inhibitors and angiotensin II type-1 receptor blockers (ARBs) inhibit the detrimental hyperactivation of the RAS by SARS-CoV-2 and increase the expression of ACE2, which is a counter-regulator of the RAS. Several studies have investigated the beneficial profile of RAS inhibitors in COVID-19; however, this finding remains unclear. Further prospective studies are warranted to confirm the role of RAS inhibitors in COVID-19. In this review, we summarize the potential effects of RAS inhibitors that have come to light thus far and review the impact of RAS inhibitors on COVID-19.
Similar content being viewed by others
ACE2, counterregulatory renin–angiotensin system axis
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by binding to angiotensin-converting enzyme 2 (ACE2) [1]. ACE2, a membrane-bound enzyme, is present in the lungs, intestine, liver, heart, testis, and kidney tissues; it is also released into the circulatory system [2]. ACE2 was discovered in 2000 and has 60% homology with angiotensin-converting enzyme 1 (ACE1). It has recently attracted increased attention owing to its association with SARS-CoV-2 [3]. SARS-CoV-2 has four main structural proteins: spike, envelope, nucleocapsid, and membrane proteins. The interaction of the spike protein of SARS-CoV-2 with the entry receptor ACE2 is the first step of SARS-CoV-2 entering host cells [1]. When SARS-CoV-2 invades the host, the spike protein is primed by transmembrane protease serine 2 (TMPRSS2), a cellular serine protease [1]. The downregulation and depletion of ACE2 results from the binding of SARS-CoV-2 to ACE2 and processing of the spike protein by TMPRSS2 [1, 4]. In addition to its pathogenic role as the entry receptor of SARS-CoV-2, the physiological essential role of ACE2 is as a pivotal counterregulatory enzyme of the renin–angiotensin system (RAS) in various cell types.
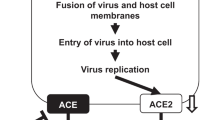
The clinical severity of COVID-19 ranges widely from asymptomatic to mild respiratory symptoms and even critical situations, with serious complications involving the cardiovascular and pulmonary systems (e.g., myocardial injury, myocarditis, myocardial infarction, acute respiratory distress syndrome [ARDS], and renal failure). ACE2 and the RAS play important pathophysiological roles in COVID-19 through the downregulation of ACE2 expression by the SARS-CoV-2 spike protein. Figure 1 illustrates the potential role of the RAS and its inhibitors in COVID-19. After engagement with the plasma membrane, SARS-CoV-2 undergoes endocytosis, resulting in reduced ACE2 expression in infected cells. ACE2 generates Ang 1–9 by cleaving a single residue from angiotensin I (Ang I) and degrades Ang II, the main effector of the RAS, to the vasodilator Ang 1–7. Therefore, ACE2 acts in a counterregulatory manner in the ACE-mediated RAS. The RAS regulates blood pressure, renal function, water homeostasis, electrolyte balance, and inflammation through the production of Ang II. In addition to the downregulation of Ang II, Ang 1–7 metabolized from Ang II by ACE2 exerts vasodilatory effects and improves cardiac function through the activation of the Ang 1–7/MAS receptor pathway. Thus, ACE2 has multiple beneficial effects on cardiovascular pathologies, diabetic injury, fibrosis, inflammation, and, most importantly, acute lung injury [5,6,7]. Although ACE2 and its receptor function as the counterregulatory axis of the RAS, the binding of SARS-CoV-2 to ACE2 and processing of the spike protein by TMPRSS2 result in the downregulation and depletion of ACE2. SARS-CoV-2 infection activates the local RAS and downregulates membrane-bound ACE2 in the lungs, contributing to more severe inflammation and ARDS.
SARS-CoV-2 Interaction with the renin–angiotensin system. A Angiotensin-converting enzyme (ACE) converts angiotensin I into angiotensin II. Angiotensin II, the main effector of the RAS, stimulates the secretion of aldosterone, which stimulates salt and water reabsorption by the kidneys, the constriction of small arteries (arterioles), and inflammation. ACE2 degrades Ang I to Ang 1–9 and Ang II to Ang 1–7. In addition to the downregulation of Ang I and Ang II, Ang 1–7 exerts functions such as vasodilation and an improvement in cardiac function through activation of the Ang 1–7/MAS receptor pathway. Therefore, ACE2 acts in a counterregulatory manner in the ACE-mediated RAS. ACE2 has multiple beneficial effects on cardiovascular pathologies, inflammation, and, most importantly, acute lung injury. B The binding of SARS-CoV-2 to ACE2 results in the downregulation and depletion of ACE2. SARS-CoV-2 infection activates the local RAS and downregulates membrane-bound ACE2 in the lungs, contributing to more severe inflammation and ARDS. C RAS inhibitors, including ACEIs and ARBs, restore and increase ACE2 expression. ACEIs and ARBs inhibit the detrimental hyperactivation of the RAS by SARS-CoV-2 and increase the expression of ACE2, which is a counterregulator of the RAS. Thus, these drugs are expected to improve COVID-19 clinical outcomes. ACE angiotensin-converting enzyme, ACE2 angiotensin-converting enzyme 2, ACEI angiotensin-converting enzyme inhibitor, Ang I angiotensin 1, Ang II angiotensin 2, Ang 1–7 angiotensin 1–7, ARB angiotensin receptor blocker, ARDS acute respiratory distress syndrome, AT1R angiotensin 2 type-1 receptor, COVID-19 coronavirus disease 2019, RAS renin–angiotensin system, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
RAS inhibitors in ACE2 and COVID-19
Previous studies have reported that ACE inhibitors (ACEIs) do not inhibit ACE2 activity, and RAS inhibitors, including ACEIs and angiotensin II type-1 receptor blockers (ARBs), restore and increase ACE2 expression [8, 9]. ACE2 functions as the entry receptor of SARS-CoV-2 and as a counterregulatory enzyme to RAS activity, which induces detrimental hyperactivation of inflammatory cytokine release and subsequent ARDS. Considering the contrasting activities of ACE2 in SARS-CoV-2 infection, the question of whether to increase ACE2 levels in tissues to inhibit the inflammatory response and prevent the aggravation of COVID-19 or to promote the reduction of tissue ACE2 levels to decrease viral entry and replication was raised. However, recent large-scale clinical studies have reported that using ACEIs or ARBs did not affect either SARS-CoV-2 infection or the frequency of COVID-19 [10,11,12]. ACEIs and ARBs are expected to improve COVID-19 clinical outcomes through the counterregulation of RAS overactivation under SARS-CoV-2 infection [13].
Kanagawa RASI COVID-19 study
We conducted a multicenter retrospective observational study (Kanagawa renin–angiotensin system inhibitor COVID-19 study) of 151 patients who were diagnosed with COVID-19 through polymerase chain reaction tests at six hospitals in Kanagawa, Japan [14]. The purpose of this study was to examine whether the preceding use of ACEIs and ARBs affected the clinical manifestations and prognoses of COVID-19 patients. Among the study population (n = 151), 39 had hypertension, and 21 had been using ACEIs or ARBs preceding COVID-19. With this small number of patients, we could not demonstrate the statistical significance of RAS inhibitors for in-hospital death or severity of the respiratory condition. However, RAS inhibitor use was significantly associated with a lower incidence of confusion in COVID-19 patients with hypertension.
Delirium, an acute state of confusion, is characterized by an altered level of consciousness and attention. Delirium is a multifactorial syndrome that is caused by complex interrelationships. In addition, delirium reflects COVID-19 disease severity. It is suggested that involvement of the brain and neuroinflammatory reactions is a key factor for acute brain dysfunction in COVID-19 patients [15,16,17]. Systemic inflammation increases blood–brain barrier permeability and alters the expression of tight junction proteins, resulting in the spread of inflammation into the central nervous system [16]. Counterregulation of RAS inhibitors to RAS overactivation in SARS-CoV-2 infection may contribute to the decreased occurrence of confusion in patients with COVID-19 [18, 19]. Delirium is a common complication of SARS-CoV-2 infection and is associated with a longer hospital stay and increased mortality [20]. A recent meta-analysis reported that the pooled prevalence of delirium was 24.3%, and it ranged widely from 2.8% to 77.4% [21]. The same study reported that the pooled incidence of delirium was 32.4%, ranging from 4.0% to 80.2%, and delirium was associated with a threefold higher mortality rate (44.5% mortality in patients with COVID-19 and delirium). A prospective observational study conducted in Italy and published in 2022 reported delirium in 28% of patients with COVID-19-related pneumonia admitted to a noncritical care unit, and delirium was associated with 7 times higher mortality in COVID-19 patients after adjusting for age and frailty [20]. In our study, 14 out of 151 COVID-19 patients (9.3%) had newly developed delirium, a rate that was relatively lower than that in previous studies, and the mortality rate in the patients with delirium was 78.6% [14]. The frequency of delirium and mortality from delirium in COVID-19 patients was reportedly lower in Asian countries than in non-Asian countries [21]. However, considering the retrospective nature of our study, the incidence of delirium was likely underestimated. The pathophysiology of the high mortality related to delirium in COVID-19 is multifactorial. In addition, delirium reflects COVID-19 severity, and the involvement of SARS-CoV-2 in the brain is suggested to be a major factor [17, 22]. Thus, in COVID-19 patients, delirium needs to be assessed regularly and routinely, and it should be recognized and managed early [23]. However, there have been no randomized clinical trials investigating the treatment or prevention of delirium in COVID-19 patients.
Randomized trials on the effects of RAS inhibitors on COVID-19
Although the association between ACE2 expression and RAS inhibitors is still being studied, the effects of RAS inhibitors on clinical outcomes in COVID-19 patients have been reported by many studies, particularly prospective randomized studies, as shown in Table 1 [24,25,26,27,28,29]. In early 2021, the REPLACE COVID trial and the BRACE CORONA trial were published [24, 25]. The REPLACE COVID trial [24] was a prospective, randomized, open-label trial that enrolled 152 patients (mean age, 62 years) who were hospitalized for COVID-19 with chronic prescription of ACEIs or ARBs prior to hospitalization. Patients were randomized into groups for the continuation or discontinuation of RAS blockers. There were no differences in the global rank score, intensive care unit (ICU) admission, invasive mechanical ventilation, or death between the two arms. The BRACE CORONA trial [25] from Brazil enrolled 659 hospitalized COVID-19 patients (median age, 55 years) who were using ACE inhibitors or ARBs prior to hospitalization. The authors compared the number of days patients survived and were out of the hospital up to 30 days after randomization into groups for the continuation or discontinuation of RAS blockers and observed no difference between the groups (discontinuation group 21.9 ± 8 days and continuation group 22.9 ± 7.1 days). In the BRACE CORONA trial, there were significant interactions between randomization and some subgroups, which indicated that the continuation of ACEIs or ARBs may be more beneficial than discontinuation in patients with lower oxygen saturation and in those with greater COVID-19 severity at hospital admission. The ACEI-COVID trial [26] was published online in June 2021. It included 204 COVID-19 patients who were on chronic ACEIs or ARBs and were randomly divided into two groups according to the continuation or discontinuation of RAS blockers. The ACEI-COVID trial included older people (median age, 75 years) than the previous two trials. Although secondary endpoints, such as the area under the receiver operating characteristic (ROC) curve for the Sequential Organ Failure Assessment (SOFA) score and mean SOFA score, were significantly lower in the discontinuation group than in the continuation group, the primary endpoint of the maximal SOFA score within 30 days was not different between the two groups.
In addition to the abovementioned randomized clinical trials that compared the continuation and discontinuation of RAS blockers in COVID-19 patients, two randomized clinical trials investigated the effect of the initiation of RAS blockers in COVID-19 patients who were not on chronic ACEIs or ARBs [28, 29]. A double-blind randomized study from Minnesota included 117 symptomatic outpatients with COVID-19 not already on ACEIs or ARBs [28]. Patients were randomly administered losartan 25 mg twice daily or a placebo for 10 days. The primary outcome, all-cause hospitalization within 15 days, did not differ between groups. Viral load, the secondary outcome, was also not significantly affected by the treatment. Duarte et al. reported an open-label randomized trial of 158 patients hospitalized for COVID-19 who were not on ACEIs or ARBs on admission [29]. Patients in the control arm received standard care only, whereas those in the treatment arm were administered a high dose of telmisartan (80 mg twice daily) in addition to standard care for 14 days. C-reactive protein levels significantly decreased from 9.04 mg/dL at baseline to 3.83 mg/dL on Day 5 and 2.37 mg/dL on Day 8 in the telmisartan group; however, in the control group, they did not decrease (5.53 mg/dL at baseline, 6.06 mg/dL on Day 5, and 6.30 mg/dL on Day 8), suggesting an anti-inflammatory effect of telmisartan in COVID-19. Furthermore, the median time to discharge in the telmisartan group was significantly shorter than that in the control group, (9 days in the telmisartan group vs. 15 days in the control group), and the 30-day mortality rate was significantly lower (4.29% in the telmisartan group vs. 22.54% in the control group, P = 0.002). The combination of ICU admission, mechanical ventilation, and death was also reduced by telmisartan treatment on Days 15 and 30. No adverse events with high-dose telmisartan were reported in this study. It is suggested that the early and sustained administration of an ARB at a high dose will be more effective against COVID-19 than the late administration of an ARB in severe cases of lung injury.
Future prospects
Since the beginning of the COVID-19 pandemic, the potential roles of RAS inhibitors in COVID-19 have been widely debated as favorable, harmful, or neutral. As mentioned above, large observational retrospective studies in humans and meta-analyses have revealed that there is no harmful effect of RAS blockers on COVID-19 susceptibility, severity, or mortality. Later, the beneficial effects of RAS inhibitors on COVID-19 were investigated; however, evidence to guide clinical decision-making is still scarce. Several randomized clinical trials investigating this issue are ongoing, and the International Society of Hypertension is conducting a prospective meta-analysis [30].
References
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80 e8.
Surjit M, Liu B, Kumar P, Chow VT, Lal SK. The nucleocapsid protein of the SARS coronavirus is capable of self-association through a C-terminal 209 amino acid interaction domain. Biochem Biophys Res Commun. 2004;317:1030–6.
Touyz RM, Li H, Delles C. ACE2 the Janus-faced protein - from cardiovascular protection to severe acute respiratory syndrome-coronavirus and COVID-19. Clin Sci. 2020;134:747–50.
Tang Q, Wang Y, Ou L, Li J, Zheng K, Zhan H, et al. Downregulation of ACE2 expression by SARS-CoV-2 worsens the prognosis of KIRC and KIRP patients via metabolism and immunoregulation. Int J Biol Sci. 2021;17:1925–39.
Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101.
Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–28.
Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77:301–8.
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–10.
Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21.
Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1020–6.
Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–40.
Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43:648–54.
McFarlane E, Linschoten M, Asselbergs FW, Lacy PS, Jedrzejewski D, Williams B, et al. The impact of pre-existing hypertension and its treatment on outcomes in patients admitted to hospital with COVID-19. Hypertens Res. 2022;45:834–45. https://doi.org/10.1038/s41440-022-00893-5.
Matsuzawa Y, Ogawa H, Kimura K, Konishi M, Kirigaya J, Fukui K, et al. Renin-angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43:1257–66.
Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7.
Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–54.
Rozzini R, Bianchetti A, Mazzeo F, Cesaroni G, Bianchetti L, Trabucchi M. Delirium: clinical presentation and outcomes in older COVID-19 patients. Front Psychiatry. 2020;11:586686.
de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–34.
Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–60.
Callea A, Conti G, Fossati B, Carassale L, Zagaria M, Caporotundo S, et al. Delirium in hospitalized patients with COVID-19 pneumonia: a prospective, cross-sectional, cohort study. Intern Emerg Med. 2022. https://doi.org/10.1007/s11739-022-02934-w.
Shao SC, Lai CC, Chen YH, Chen YC, Hung MJ, Liao SC. Prevalence, incidence and mortality of delirium in patients with COVID-19: a systematic review and meta-analysis. Age Ageing. 2021;50:1445–53.
McLoughlin BC, Miles A, Webb TE, Knopp P, Eyres C, Fabbri A, et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020;11:857–62.
Duggan MC, Van J, Ely EW. Delirium assessment in critically ill older adults: considerations during the COVID-19 pandemic. Crit Care Clin. 2021;37:175–90.
Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-Santander NR, Medina C, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–84.
Lopes RD, Macedo AVS, de Barros E, Silva PGM, Moll-Bernardes RJ, Dos Santos TM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–64.
Bauer A, Schreinlechner M, Sappler N, Dolejsi T, Tilg H, Aulinger BA, et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med. 2021;9:863–72.
Najmeddin F, Solhjoo M, Ashraf H, Salehi M, Rasooli F, Ghoghaei M, et al. Effects of renin-angiotensin-aldosterone inhibitors on early outcomes of hypertensive COVID-19 patients: a randomized triple-blind clinical trial. Am J Hypertens. 2021;34:1217–26.
Puskarich MA, Cummins NW, Ingraham NE, Wacker DA, Reilkoff RA, Driver BE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37:100957.
Duarte M, Pelorosso F, Nicolosi LN, Salgado MV, Vetulli H, Aquieri A, et al. Telmisartan for treatment of COVID-19 patients: an open multicenter randomized clinical trial. EClinicalMedicine. 2021;37:100962.
Gnanenthiran SR, Borghi C, Burger D, Charchar F, Poulter NR, Schlaich MP, et al. Prospective meta-analysis protocol on randomised trials of renin-angiotensin system inhibitors in patients with COVID-19: an initiative of the International Society of Hypertension. BMJ Open. 2021;11:e043625.
Acknowledgements
We are particularly indebted to the researchers of the Kanagawa RASI COVID-19 study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsuzawa, Y., Kimura, K., Ogawa, H. et al. Impact of renin–angiotensin–aldosterone system inhibitors on COVID-19. Hypertens Res 45, 1147–1153 (2022). https://doi.org/10.1038/s41440-022-00922-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-00922-3
Keywords
This article is cited by
-
2023 update and perspectives
Hypertension Research (2024)
-
Mitigating secondary disaster triggered by fear of COVID-19: the role of professional medical societies
Hypertension Research (2023)
-
Prognostic significance of hypertension history and blood pressure on admission in Japanese patients with coronavirus disease 2019: integrative analysis from the Japan COVID-19 Task Force
Hypertension Research (2023)