Abstract
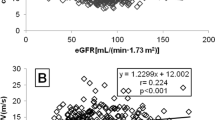
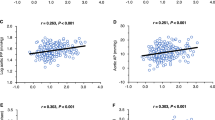
Although central hemodynamics are known to be closely associated with microvascular damage, their association with lesions in the small renal arteries has not yet been fully clarified. We focused on arterioles in renal biopsy specimens and analyzed whether their structural changes were associated with noninvasive vascular function parameters, including central blood pressure (BP) and brachial-ankle pulse wave velocity (baPWV). Forty-four nondiabetic patients (18–50 years of age) with preserved renal function underwent renal biopsy. Wall thickening of arterioles was analyzed based on the media/diameter ratio, and hyalinosis was analyzed by semiquantitative grading. Associations of these indexes (arteriolar wall remodeling grade index (RG index) and arteriolar hyalinosis index (Hyl index)) with clinical variables were analyzed. Multiple regression analyses demonstrated that the RG index was significantly associated with central systolic BP (β = 0.97, p = 0.009), serum cystatin C-based estimated glomerular filtration rate (β = −0.36, p = 0.04), and high-density lipoprotein cholesterol levels (β = −0.37, p = 0.02). The Hyl index was significantly associated with baPWV (β = 0.75, p = 0.01). Our results indicate that aortic stiffness and abnormal central hemodynamics are closely associated with renal microvascular damage in young to middle-aged, nondiabetic kidney disease patients with preserved renal function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–60.
Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006;48:1012–7.
Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. 2013;2013:808353.
Hill GS, Heudes D, Jacquot C, Gauthier E, Bariéty J. Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int. 2006;69:823–31.
Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Katafuchi R, Hirakata H, et al. Risk factors for renal glomerular and vascular changes in an autopsy-based population survey: the Hisayama study. Kidney Int. 2003;63:1508–15.
Tracy RE, MacLean CJ, Reed DM, Hayashi T, Gandia M, Strong JP. Blood pressure, nephrosclerosis, and age autopsy findings from the Honolulu Heart Program. Mod Pathol. 1988;1:420–7.
Ikee R, Kobayashi S, Saigusa T, Namikoshi T, Yamada M, Hemmi N, et al. Impact of hypertension and hypertension-related vascular lesions in IgA nephropathy. Hypertens Res. 2006;29:15–22.
Zhang Y, Sun L, Zhou S, Xu Q, Xu Q, Liu D, et al. Intrarenal arterial lesions are associated with higher blood pressure, reduced renal function and poorer renal outcomes in patients with IgA nephropathy. Kidney Blood Press Res. 2018;43:639–50.
Shimizu M, Furuichi K, Toyama T, Kitajima S, Hara A, Kitagawa K, et al. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care. 2013;36:3655–62.
Moriya T, Omura K, Matsubara M, Yoshida Y, Hayama K, Ouchi M. Arteriolar hyalinosis predicts increase in albuminuria and GFR decline in normo- and microalbuminuric Japanese patients with type 2 diabetes. Diabetes Care. 2017;40:1373–8.
McGill HC Jr., Strong JP, Tracy RE, McMahan CA, Oalmann MC. Relation of a postmortem renal index of hypertension to atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 1995;15:2222–8.
Burchfiel CM, Tracy RE, Chyou PH, Strong JP. Cardiovascular risk factors and hyalinization of renal arterioles at autopsy. The Honolulu Heart Program. Arterioscler Thromb Vasc Biol. 1997;17:760–8.
Ochi N, Kohara K, Tabara Y, Nagai T, Kido T, Uetani E, et al. Association of central systolic blood pressure with intracerebral small vessel disease in Japanese. Am J Hypertens. 2010;23:889–94.
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71.
Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens. 2010;28:384–8.
Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, et al. Arterial remodeling associates with CKD progression. J Am Soc Nephrol. 2011;22:967–74.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–6.
Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–7.
Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, et al. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol. 2015;26:1181–7.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61:197–203.
Namikoshi T, Fujimoto S, Yorimitsu D, Ihoriya C, Fujimoto Y, Komai N, et al. Relationship between vascular function indexes, renal arteriolosclerosis, and renal clinical outcomes in chronic kidney disease. Nephrology. 2015;20:585–90.
Kohagura K, Kochi M, Miyagi T, Kinjyo T, Maehara Y, Nagahama K, et al. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: a biopsy-based study. Hypertens Res. 2013;36:43–9.
Bader H, Meyer DS. The size of the juxtaglomerular apparatus in diabetic glomerulosclerosis and its correlation with arteriolosclerosis and arterial hypertension: a morphometric light microscopic study on human renal biopsies. Clin Nephrol. 1977;8:308–11.
Prewitt RL, Chen II, Dowell RF. Microvascular alterations in the one-kidney, one-clip renal hypertensive rat. Am J Physiol. 1984;246:H728–32.
Grassi G, Seravalle G, Scopelliti F, Dell’Oro R, Fattori L, Quarti-Trevano F, et al. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity. 2010;18:92–8.
Ikee R, Honda K, Ishioka K, Oka M, Maesato K, Moriya H, et al. Postprandial hyperglycemia and hyperinsulinemia associated with renal arterio-arteriolosclerosis in chronic kidney disease. Hypertens Res. 2010;33:499–504.
Miyagi T, Kohagura K, Ishiki T, Kochi M, Kinjyo T, Kinjyo K, et al. Interrelationship between brachial artery function and renal small artery sclerosis in chronic kidney disease. Hypertens Res. 2014;37:863–9.
Biava CG, Dyrda I, Genest J, Bencosme SA. Renal hyaline arteriolosclerosis. an electron microscope study. Am J Pathol. 1964;44:349–63.
Snanoudj R, Royal V, Elie C, Rabant M, Girardin C, Morelon E, et al. Specificity of histological markers of long-term CNI nephrotoxicity in kidney-transplant recipients under low-dose cyclosporine therapy. Am J Transplant. 2011;11:2635–46.
Hill GS, Heudes D, Bariéty J. Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation. Kidney Int. 2003;63:1027–36.
Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601.
Zamami R, Kohagura K, Miyagi T, Kinjyo T, Shiota K, Ohya Y. Modification of the impact of hypertension on proteinuria by renal arteriolar hyalinosis in nonnephrotic chronic kidney disease. J Hypertens. 2016;34:2274–9.
Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839–46.
Tsioufis C, Tzioumis C, Marinakis N, Toutouzas K, Tousoulis D, Kallikazaros I, et al. Microalbuminuria is closely related to impaired arterial elasticity in untreated patients with essential hypertension. Nephron Clin Pract. 2003;93:c106–11.
Hashimoto J, Ito S. Aortic blood flow reversal determines renal function: potential explanation for renal dysfunction caused by aortic stiffening in hypertension. Hypertension. 2015;66:61–7.
Takase H, Dohi Y, Kimura G. Distribution of central blood pressure values estimated by Omron HEM-9000AI in the Japanese general population. Hypertens Res. 2013;36:50–7.
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–50.
Zamami R, Ishida A, Miyagi T, Yamazato M, Kohagura K, Ohya Y. A high normal ankle-brachial index is associated with biopsy-proven severe renal small artery intimal thickening and impaired renal function in chronic kidney disease. Hypertens Res. 2020;43:929–37.
Hashimoto J. Central hemodynamics for management of arteriosclerotic diseases. J Atheroscler Thromb. 2017;24:765–78.
Nordstrand N, Gjevestad E, Hertel JK, Johnson LK, Saltvedt E, Røislien J, et al. Arterial stiffness, lifestyle intervention and a low-calorie diet in morbidly obese patients-a nonrandomized clinical trial. Obesity. 2013;21:690–7.
Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–7.
Hashimoto J, Imai Y, O’Rourke MF. Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens. 2007;20:378–84.
Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O’Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–24.
Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, et al. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: a meta-analysis of individual data in 294 patients. J Hypertens. 2011;29:1034–42.
Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–7.
Acknowledgements
The authors are indebted to Dr. Helena Akiko Popiel of the Department of International Medical Communications at Tokyo Medical University for her editorial review of the English manuscript. We also wish to thank Dr. Tatsuya Isomura of the Department of Clinical Consultation, Medical Research Institute, Tokyo Medical University, for providing statistical advice and the nephrologists of Tokyo Medical University for ensuring compliance with clinical study protocols.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miyaoka, Y., Okada, T., Tomiyama, H. et al. Structural changes in renal arterioles are closely associated with central hemodynamic parameters in patients with renal disease. Hypertens Res 44, 1113–1121 (2021). https://doi.org/10.1038/s41440-021-00656-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00656-8
Keywords
This article is cited by
-
Aerobic exercise improves central blood pressure and blood pressure variability among patients with resistant hypertension: results of the EnRicH trial
Hypertension Research (2023)
-
Update on Hypertension Research in 2021
Hypertension Research (2022)