Abstract
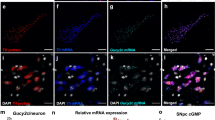
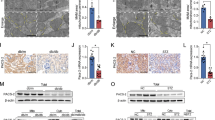
Overproduction of reactive oxygen species (ROS) plays an important role in the pathogenesis of hypertension. The dopamine D5 receptor (D5R) is known to decrease ROS production, but the mechanism is not completely understood. In HEK293 cells overexpressing D5R, fenoldopam, an agonist of the two D1-like receptors, D1R and D5R, decreased the production of mitochondria-derived ROS (mito-ROS). The fenoldopam-mediated decrease in mito-ROS production was mimicked by Sp-cAMPS but blocked by Rp-cAMPS. In human renal proximal tubule cells with DRD1 gene silencing to eliminate the confounding effect of D1R, fenoldopam still decreased mito-ROS production. By contrast, Sch23390, a D1R and D5R antagonist, increased mito-ROS production in the absence of D1R, D5R is constitutively active. The fenoldopam-mediated inhibition of mito-ROS production may have been related to autophagy because fenoldopam increased the expression of the autophagy hallmark proteins, autophagy protein 5 (ATG5), and the microtubule-associated protein 1 light chain (LC)3-II. In the presence of chloroquine or spautin-1, inhibitors of autophagy, fenoldopam further increased ATG5 and LC3-II expression, indicating an important role of D5R in the positive regulation of autophagy. However, when autophagy was inhibited, fenoldopam was unable to inhibit ROS production. Indeed, the levels of these autophagy hallmark proteins were decreased in the kidney cortices of Drd5−/− mice. Moreover, ROS production was increased in mitochondria isolated from the kidney cortices of Drd5−/− mice, relative to Drd5+/+ littermates. In conclusion, D5R-mediated activation of autophagy plays a role in the D5R-mediated inhibition of mito-ROS production in the kidneys.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal. 2014;20:74–101.
Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am. 2017;101:169–93.
Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol. 2015;31:631–41.
Addabbo F, Montagnani M, Goligorsky MS. Mitochondria and reactive oxygen species. Hypertension. 2009;53:885–92.
Zhang MZ, Harris RC. Antihypertensive mechanisms of intra-renal dopamine. Curr Opin Nephrol Hypertens. 2015;24:117–22.
Asghar M, Tayebati SK, Lokhandwala MF, Hussain T. Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep. 2011;13:294–302.
Zeng C, Jose PA. Dopamine receptors: important antihypertensive counterbalance against hypertensive factors. Hypertension. 2011;57:11–7.
Allayee H, de Bruin TW, Michelle Dominguez K, Cheng LS, Ipp E, Cantor RM, et al. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–8.
Cohn DH, Shohat T, Yahav M, Ilan T, Rechavi G, King L, et al. A locus for an autosomal dominant form of progressive renal failure and hypertension at chromosome 1q21. Am J Hum Genet. 2000;67:647–51.
Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–104.
Saez F, Hong NJ, Garvin JL. Luminal flow induces NADPH oxidase 4 translocation to the nuclei of thick ascending limbs. Physiol Rep. 2016;4:e12724.
Yang Q, Wu FR, Wang JN, Gao L, Jiang L, Li HD, et al. Nox4 in renal diseases: an update. Free Radic Biol Med. 2018;124:466–72.
Haque MZ, Majid DS. Reduced renal responses to nitric oxide synthase inhibition in mice lacking the gene for gp91phox subunit of NAD(P)H oxidase. Am J Physiol Ren Physiol. 2008;295:F758–64.
Ryter SW, Bhatia D, Choi ME. Autophagy: a lysosome-dependent process with implications in cellular redox homeostasis and human disease. Antioxid Redox Signal. 2019;30:138–59.
Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75.
Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–64.
Woodall BP, Gustafsson AB. Autophagy—a key pathway for cardiac health and longevity. Acta Physiol. 2018;20:e13074.
Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I. Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. FEBS J. 2017;284:42–55.
Peña-Oyarzun D, Bravo-Sagua R, Diaz-Vega A, Aleman L, Chiong M, Garcia L, et al. Autophagy and oxidative stress in non-communicable diseases: a matter of the inflammatory state. Free Radic Biol Med. 2018;124:61–78.
Wible DJ, Bratton SB. Reciprocity in ROS and autophagic signaling. Curr Opin Toxicol. 2018;7:28–36.
Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension. 2008;51:360–6.
Jean-Charles PY, Snyder JC, Shenoy SK. Ubiquitination and deubiquitination of G protein-coupled receptors. Prog Mol Biol Transl Sci. 2016;141:1–55.
Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Investig. 2008;118:2180–9.
Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, et al. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10.
Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, et al. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–42.
O’Connell DP, Botkin SJ, Ramos SI, Sibley DR, Ariano MA, Felder RA, et al. Localization of dopamine D1A receptor protein in rat kidneys. Am J Physiol. 1995;268:F1185–97.
Ennis RC, Asico LD, Armando I, Yang J, Feranil JB, Jurgens JA, et al. Dopamine D1-like receptors regulate the α1A-adrenergic receptor in human renal proximal tubule cells and D1-like dopamine receptor knockout mice. Am J Physiol Ren Physiol. 2014;307:F1238–48.
Wang X, Li F, Jose PA, Ecelbarger CM. Reduction of renal dopamine receptor expression in obese Zucker rats: role of sex and angiotensin II. Am J Physiol Ren Physiol. 2010;299:F1164–70.
Li H, Li HF, Felder RA, Periasamy A, Jose PA. Actin cytoskeleton-dependent Rab GTPase-regulated angiotensin type I receptor lysosomal degradation studied by fluorescence lifetime imaging microscopy. J Biomed Opt. 2008;13:031206.
Lee H, Abe Y, Lee I, Shrivastav S, Crusan AP, Huttemann M, et al. Increased mitochondrial activity in renal proximal tubule cells from young spontaneously hypertensive rats. Kidney Int. 2014;85:561–9.
Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, et al. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–100.
Polster BM, Nicholls DG, Ge SX, Roelofs BA. Use of potentiometric fluorophores in the measurement of mitochondrial reactive oxygen species. Methods Enzymol. 2014;547:225–50.
Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys. 2004;431:138–44.
Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev. 2006;24:261–74.
Gimenez-Xavier P, Francisco R, Santidrian AF, Gil J, Ambrosio S. Effects of dopamine on LC3-II activation as a marker of autophagy in a neuroblastoma cell model. Neurotoxicology. 2009;30:658–65.
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–55.
Kirkman DL, Muth BJ, Ramick MG, Townsend RR, Edwards DG. Role of mitochondria-derived reactive oxygen species in microvascular dysfunction in chronic kidney disease. Am J Physiol Ren Physiol. 2018;314:F423–9.
Bonora M, Wieckowski MR, Sinclair DA, Kroemer G, Pinton P, Galluzzi L. Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles. Nat Rev Cardiol. 2019;16:33–55.
Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, et al. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res. 2016;119:e39–75.
Lee R, Margaritis M, Channon KM, Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem. 2012;19:2504–20.
Mason RP. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol. 2016;8:422–9.
Kalyanaraman B, Dranka BP, Hardy M, Michalski R, Zielonka J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes–the ultimate approach for intra- and extracellular superoxide detection. Biochim Biophys Acta. 2014;1840:739–44.
Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA. 2006;103:15038–43.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Bleier L, Drose S. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta. 2013;1827:1320–31.
Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem. 2017;292:16804–9.
Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, et al. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharm. 2017;174:1670–89.
Li H, Han W, Villar VA, Keever LB, Lu Q, Hopfer U, et al. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension. 2009;53:1054–61.
Yang S, Yang Y, Yu P, Yang J, Jiang X, Villar VA, et al. Dopamine D1 and D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free Radic Res. 2015;49:397–410.
Sulaiman D, Li J, Devarajan A, Cunningham CM, Li M, Fishbein GA, et al. Paraoxonase 2 protects against acute myocardial ischemia-reperfusion injury by modulating mitochondrial function and oxidative stress via the PI3K/Akt/GSK-3β RISK pathway. J Mol Cell Cardiol. 2019;129:154–64.
Li Z, Ji X, Wang W, Liu J, Liang X, Wu H, et al. Ammonia induces autophagy through dopamine receptor D3 and mTOR. PLoS ONE. 2016;11:e0153526.
Shin JH, Park SJ, Kim ES, Jo YK, Hong J, Cho DH. Sertindole, a potent antagonist at dopamine D2 receptors, induces autophagy by increasing reactive oxygen species in SH-SY5Y neuroblastoma cells. Biol Pharm Bull. 2012;35:1069–75.
Yan H, Li WL, Xu JJ, Zhu SQ, Long X, Che JP. D2 dopamine receptor antagonist raclopride induces non-canonical autophagy in cardiac myocytes. J Cell Biochem. 2013;114:103–10.
Dolma S, Selvadurai HJ, Lan X, Lee L, Kushida M, Voisin V, et al. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29:859–73.
Jose PA, Eisner GM, Drago J, Carey RM, Felder RA. Dopamine receptor signaling defects in spontaneous hypertension. Am J Hypertens. 1996;9:400–5.
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225.
Gildea JJ, Shah I, Weiss R, Casscells ND, McGrath HE, Zhang J, et al. HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling. Hypertension. 2010;56:505–11.
Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–40.
Pyo JO, Nah J, Kim HJ, Lee HJ, Heo J, Lee H, et al. Compensatory activation of ERK1/2 in Atg5-deficient mouse embryo fibroblasts suppresses oxidative stress-induced cell death. Autophagy. 2008;4:315–21.
Tian Y, Kuo CF, Sir D, Wang L, Govindarajan S, Petrovic LM, et al. Autophagy inhibits oxidative stress and tumor suppressors to exert its dual effect on hepatocarcinogenesis. Cell Death Differ. 2015;22:1025–34.
Diakopoulos KN, Lesina M, Wormann S, Song L, Aichler M, Schild L, et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–38.
Harada S, Nakagawa T, Yokoe S, Edogawa S, Takeuchi T, Inoue T, et al. Autophagy deficiency diminishes indomethacin-induced intestinal epithelial cell damage through activation of the ERK/Nrf2/HO-1 pathway. J Pharm Exp Ther. 2015;355:353–61.
Jones DC, Gunasekar PG, Borowitz JL, Isom GE. Dopamine-induced apoptosis is mediated by oxidative stress and is enhanced by cyanide in differentiated PC12 cells. J Neurochem. 2000;74:2296–304.
Leng ZG, Lin SJ, Wu ZR, Guo YH, Cai L, Shang HB, et al. Activation of DRD5 (dopamine receptor D5) inhibits tumor growth by autophagic cell death. Autophagy. 2017;13:1404–19.
Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, et al. Ang II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010;299:C488–96.
Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–46.
Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, et al. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–10.
Haller M, Hock AK, Giampazolias E, Oberst A, Green DR, Debnath J, et al. Ubiquitination and proteasomal degradation of ATG12 regulates its proapoptotic activity. Autophagy. 2014;10:2269–78.
Jiang S, Park DW, Gao Y, Ravi S, Darley-Usmar V, Abraham E, et al. Participation of proteasome-ubiquitin protein degradation in autophagy and the activation of amp-activated protein kinase. Cell Signal. 2015;27:1186–97.
Livnat-Levanon N, Glickman MH. Ubiquitin-proteasome system and mitochondria - reciprocity. Biochim Biophys Acta. 2011;1809:80–7.
Omar B, Zmuda-Trzebiatowska E, Manganiello V, Göransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21:760–6.
Decara J, Rivera P, Arrabal S, Vargas A, Serrano A, Pavón FJ, et al. Cooperative role of the glucagon-like peptide-1 receptor and β3-adrenergic-mediated signalling on fat mass reduction through the downregulation of PKA/AKT/AMPK signalling in the adipose tissue and muscle of rats. Acta Physiol. 2018;222:e13008.
Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. cAMP and mitochondria. Physiology. 2013;28:199–209.
Torres-Quiroz F, Filteau M, Landry CR. Feedback regulation between autophagy and PKA. Autophagy. 2015;11:1181–3.
Lee YJ, Shu MS, Kim JY, Kim YH, Sim KH, Sung WJ, et al. Cilostazol protects hepatocytes against alcohol-induced apoptosis via activation of AMPK pathway. PLoS ONE. 2019;14:e0211415.
Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy. 2013;9:2033–45.
Akabane S, Uno M, Tani N, Shimazaki S, Ebara N, Kato H, et al. PKA regulates PINK1 stability and parkin recruitment to damaged mitochondria through phosphorylation of MIC60. Mol Cell. 2016;62:371–84.
Wolter S, Kloth C, Golombek M, Dittmar F, Försterling L, Seifert R. cCMP causes caspase-dependent apoptosis in mouse lymphoma cell lines. Biochem Pharm. 2015;98:119–31.
Yu P, Sun M, Villar VA, Zhang Y, Weinman EJ, Felder RA, et al. Differential dopamine receptor subtype regulation of adenylyl cyclases in lipid rafts in human embryonic kidney and renal proximal tubule cells. Cell Signal. 2014;26:2521–9.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (HL074940, HL023081, DK039308, HL092196, DK119652, and DK090918), the Children’s National Medical Center Intramural Avery Award, the National Natural Science Foundation of China (81670698, 91739119, 81670406, 30971186), the Second Hospital of Dalian Medical University Start-up Funds, and the Department of Education of Liaoning Province Grant (L2016020).
Author information
Authors and Affiliations
Contributions
HL and PAJ designed the experiments. HL, XJ, IP, PY, SR, JW, and YW performed the experiments. DRS supplied the mice. HL, PY, MH, RAF, BMP, IA, ZY, PQ, and PAJ interpreted the experimental results. HL and PAJ drafted the manuscript. All authors edited, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, H., Jiang, X., Perwaiz, I. et al. Dopamine D5 receptor-mediated decreases in mitochondrial reactive oxygen species production are cAMP and autophagy dependent. Hypertens Res 44, 628–641 (2021). https://doi.org/10.1038/s41440-021-00646-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00646-w
Keywords
This article is cited by
-
Dopamine and dopamine receptor D1 as a novel favourable biomarker for hepatocellular carcinoma
Cancer Cell International (2021)