Abstract
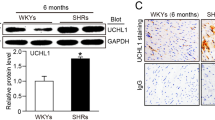
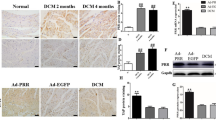
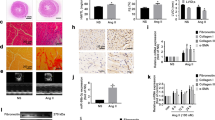
Renalase, a novel flavoprotein that is mainly expressed in the kidney and heart, plays a crucial role in hypertension. Recent studies have shown that renalase is expressed at low levels in the serum of patients with heart failure, while the role of renalase and its mechanism in cardiac failure is unclear. Adult Sprague-Dawley (SD) rats were used to investigate the role and function of renalase in the pathological process of transverse aortic constriction (TAC)-induced heart failure. Renalase-human protein chip analysis showed that renalase was directly associated with P38 and extracellular signal-regulated protein kinase 1/2 (ERK1/2) signaling. We further used lentivirus-mediated RNA interference to study the role of renalase in the progression of pathological ventricular hypertrophy and found that renalase inhibition attenuated the noradrenaline-induced hypertrophic response in vitro or the pressure overload-induced hypertrophic response in vivo. Recombinant renalase protein significantly alleviated pressure overload-induced cardiac failure and was associated with P38 and ERK1/2 signaling. These findings demonstrate that renalase is a potential biomarker of hypertrophy and that exogenous recombinant renalase is a potential and novel drug for heart failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lewington S, Lacey B, Clarke R, Guo Y, Kong XL, Yang L, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524–32.
Pitt B. Mark Nicholls speaks to Professor Bertram Pitt and his role as a pioneer in cardiology over the management of heart failure. Eur Heart J. 2020;41:1615–7.
Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–73.
Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26:343–9.
Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–62.
Bombelli M, Facchetti R, Carugo S, Madotto F, Arenare F, Quarti-Trevano F, et al. Left ventricular hypertrophy increases cardiovascular risk independently of in-office and out-of-office blood pressure values. J Hypertens. 2009;27:2458–64.
Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–80.
Luft FC. Renalase, a catecholamine-metabolizing hormone from the kidney. Cell Metab. 2005;1:358–60.
Li G, Xu J, Wang P, Velazquez H, Li Y, Wu Y, et al. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation. 2008;117:1277–82.
Desir GV, Wang L, Peixoto AJ. Human renalase: a review of its biology, function, and implications for hypertension. J Am Soc Hypertens. 2012;6:417–26.
Desir GV. Regulation of blood pressure and cardiovascular function by renalase. Kidney Int. 2009;76:366–70.
Wu Y, Xu J, Velazquez H, Wang P, Li G, Liu D, et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. 2011;79:853–60.
Lee IT, Sheu WH. Serum renalase levels are predicted by brain-derived neurotrophic factor and associated with cardiovascular events and mortality after percutaneous coronary intervention. J Clin Med. 2018;7:437.
Safdar B, Guo X, Johnson C, D’Onofrio G, Dziura J, Sinusas AJ, et al. Elevated renalase levels in patients with acute coronary microvascular dysfunction - a possible biomarker for ischemia. Int J Cardiol. 2019;279:155–61.
Li X, Xie Z, Lin M, Huang R, Liang Z, Huang W, et al. Renalase protects the cardiomyocytes of Sprague-Dawley rats against ischemia and reperfusion injury by reducing myocardial cell necrosis and apoptosis. Kidney Blood Press Res. 2015;40:215–22.
Guo X, Hollander L, MacPherson D, Wang L, Velazquez H, Chang J, et al. Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer. Sci Rep. 2016;6:22996.
Hollander L, Guo X, Velazquez H, Chang J, Safirstein R, Kluger H, et al. Renalase expression by melanoma and tumor-associated macrophages promotes tumor growth through a STAT3-mediated mechanism. Cancer Res. 2016;76:3884–94.
Stojanovic D, Mitic V, Petrovic D, Stojanovic M, Ignjatovic A, Stefanovic N, et al. Association of plasma renalase and left ventricle mass index in heart failure patients stratified to the category of the ejection fraction: a pilot study. Dis Markers. 2019;2019:7265160.
Stojanovic D, Mitic V, Stojanovic M, Petrovic D, Ignjatovic A, Stefanovic N, et al. The partnership between renalase and ejection fraction as a risk factor for increased cardiac remodeling biomarkers in chronic heart failure patients. Curr Med Res Opin. 2020;36:909–19.
Li X, Lin M, Xie Z, Huang R, Chen AF, Jiang W. Establishing a low-expression renalase gene model in cardiac tissue of Sprague-Dawley rats. Herz. 2016;41:326–30.
Li X, Jiang W, Li L, Huang R, Yang Q, Yang Y, et al. Renalase gene polymorphism in patients with hypertension and concomitant coronary heart disease. Kidney Blood Press Res. 2014;39:9–16.
Wybraniec MT, Wieczorek J, Wozniak-Skowerska I, Hoffmann A, Nowak S, Cichon M, et al. Renalase is associated with adverse left atrial remodelling and disease burden in patients with atrial fibrillation undergoing pulmonary vein isolation. Kardiol Pol. 2018;76:1232–41.
Schlaich MP, Lambert GW, Eikelis N. Renalase - a potential biomarker for risk of atrial fibrillation? Kardiol pol. 2018;76:1201–2.
Jiang W, Tan L, Guo Y, Li X, Tang X, Yang K. Effect of renal denervation procedure on left ventricular hypertrophy of hypertensive rats and its mechanisms. Acta Cir Bras. 2012;27:815–20.
Wang L, Velazquez H, Chang J, Safirstein R, Desir GV. Identification of a receptor for extracellular renalase. Plos ONE. 2015;10:e122932.
Wang Y, Safirstein R, Velazquez H, Guo XJ, Hollander L, Chang J, et al. Extracellular renalase protects cells and organs by outside-in signalling. J Cell Mol Med. 2017;21:1260–5.
Hoag MR, Roman J, Beaupre BA, Silvaggi NR, Moran GR. Bacterial renalase: structure and kinetics of an enzyme with 2- and 6-Dihydro-beta-NAD(P) oxidase activity from pseudomonas phaseolicola. Biochemistry-US. 2015;54:3791–802.
Zhao L, Cheng G, Jin R, Afzal MR, Samanta A, Xuan YT, et al. Deletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunction. Circ Res. 2016;118:1918–29.
Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57.
Miao R, Lu Y, Xing X, Li Y, Huang Z, Zhong H, et al. Regulator of G-protein signaling 10 negatively regulates cardiac remodeling by blocking mitogen-activated protein kinase-extracellular signal-regulated protein kinase 1/2 signaling. Hypertension. 2016;67:86–98.
Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, et al. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappaB. Circulation. 2012;126:418–29.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (NSFC) Projects (Nos. 81800271 and 81670335) and the Natural Science Foundation of Hunan Province (No. 2019JJ50920).
Author information
Authors and Affiliations
Contributions
XL and WJ provided the general outline of the manuscript and designed the research study, XL and YW performed all experiments and wrote the manuscript, and CQ and YY verified and analyzed the raw data after the experiments. ZL provided assistance with our animal experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, Y., Quan, C., Yang, Y. et al. Renalase improves pressure overload-induced heart failure in rats by regulating extracellular signal-regulated protein kinase 1/2 signaling. Hypertens Res 44, 481–488 (2021). https://doi.org/10.1038/s41440-020-00599-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-00599-6
Keywords
This article is cited by
-
Denervation or stimulation? Role of sympatho-vagal imbalance in HFpEF with hypertension
Hypertension Research (2023)
-
Update on Hypertension Research in 2021
Hypertension Research (2022)