Abstract
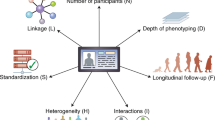
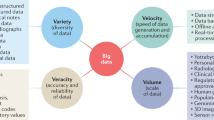
Big data has been a hot topic in medical and healthcare research. Big data in healthcare is considered to comprise massive amounts of information from various sources, including electronic health records (EHRs), administrative or claims data, and data from self-monitoring devices. Biomedical research has also generated a significant portion of big data relevant to healthcare. Other large datasets arise from cohorts that are recruited and followed on the basis of specific questions, although such research questions may later be expanded to enable other investigations. While the availability of big data offers many possibilities for an improved understanding of disease and treatment, the need for careful and productive use of statistical concepts should be kept in mind. Patient data routinely collected via electronic means are called real-world data (RWD) and are becoming common in healthcare research. RWD and big data are not synonymous with each other, but the two terms seem to be used without distinction with respect to observational studies. In this article, we review hypertension-related papers that use big data or RWD. There are many other sources of big data or RWD that are not covered here, each of which may pose special challenges and opportunities. While randomized clinical trials (RCTs) are considered to be the criterion standard for generating clinical evidence, the use of real-world evidence (RWE) to evaluate the efficacy and safety of medical interventions is gaining interest. On-going efforts to make use of RWD to generate RWE for regulatory decisions, as well as the challenges confronted, including reliability (quality) and relevance (fitness for purpose) of data, will also be addressed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Baro E, Degoul S, Beuscart R, Chazard E. Toward a literature-driven definition of big data in healthcare. Biomed Res Int. 2015:639021. https://doi.org/10.1155/2015/639021.
Conroy M, Sellors J, Effingham M, Littlejohns TJ, Boultwood C, Gillions L, et al. The advantages of UK Biobank’s open-access strategy for health research. J Intern Med. 2019;286:389–97.
Cox DR, Kartsonaki C, Keogh RH. Big data: some statistical issues. Stat Probab Lett. 2018;136:111–5.
Shilo S, Rossman H, Segal E. Axes of a revolution: challenges and promises of big data in healthcare. Nat Med. 2020;26:29–38.
U.S. Food and Drug Administration. Framework for FDA’s real-world evidence programa. 2018. https://www.fda.gov/media/120060/download.
Stevens SS, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354. https://doi.org/10.1136/bmj.i4098.
Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;27:1375–86.
Liu J, Ma J, Wang J, Zeng DD, Song H, Wang L, et al. Comorbidity analysis according to sex and age in hypertension patients in China. Int J Med Sci. 2016;13:99–107.
Son JS, Choi S, Kim K, Kim SM, Choi D, et al. Association of blood pressure classification in Korean young adults according to the 2017 American College of Cardiology/American Heart Association Guidelines with subsequent cardiovascular disease events. JAMA. 2018;320:1783–92.
Lee H, Park JH, Floyd JS, Park S, Kim HC. Combined effect of income and medication adherence on mortality in newly treated hypertension: nationwide study of 16 million person-years. J Am Heart Assoc. 2019;8:e013148.
Schaffer AL, Zoega H, Tran DT, Buckley NA, Pearson S, Havard A. Trajectories of antipsychotic use before and during pregnancy and associated maternal and birth characteristics. Aust N. Z J Psychiatry. 2019;53:1208–21.
Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR, et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet. 2019;51:51–62.
Warren HR, Evangelou E, Cabrera CP, Gao H, Ren M, Mifsud B, et al. Corrigendum: Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. 2017;49:403–15.
Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–25.
Doherty A, Smith-Byrne K, Ferreira T, Holmes MV, Holmes C, Pulit SL, et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun. 2018;9:5257.
Bartlett VB, Dhruva SS, Shah ND, Ryan P, Ross JS. Feasibility of using real-world data to replicate clinical trial evidence. JAMA Netw Open. 2019;2:e1912869.
Sison J, Vega RMR, Dayi H, Bader G, P Brunel. PEfficacy and effectiveness of valsartan/amlodipine and valsartan/amlodipine/hydrochlorothiazide in hypertension: randomized controlled versus observational studies. Curr Med Res Opin. 2018;34:501–15.
Suchard MA, Schuemie MJ, Krumholz HM, You SC, Chen RJ, Pratt N, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394:1816–26.
Sabidó M, Hohenberger T, Grassi G. Pharmacological intervention in hypertension using beta-blockers: real-world evidence for long-term effectiveness. Pharm Res. 2018;130:191–7.
Marinier K, Macouillard P, de Champvallins M, Deltour N, Poulter N, Mancia G. Effectiveness of two-drug therapy versus monotherapy as initial regimen in hypertension: a propensity score-matched cohort study in the UK clinical practice research datalink. Pharmacoepidemiol Drug Saf. 2019 ;28:1572–82.
Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Health. 2016;9:211–7.
Matsumoto S, Fukui M, Hamaguchi M, Ushigome E, Matsushita K, Fukuda T, et al. Is home blood pressure reporting in patients with type 2 diabetes reliable? Hypertension Res. 2014;37:741–5.
Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–22.
The International Council for Harmonization. ICH reflection on “GCP Renovation”: modernization of ICH E8 and subsequent renovation of ICH E6. 2017. https://admin.ich.org/sites/default/files/inline-files/ICH_Reflection_paper_GCP_Renovation_Jan_2017 _Final.pdf.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP.STROBE Initiative Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–4.
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885.
Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ. 2018;363:k3532.
The International Society for Pharmacoepidemiology (ISPE). The position on RWE. 2020. https://pharmacoepi.org/pub/?id=136DECF1-C559-BA4F-92C4-CF6E3ED16BB6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okada, M. Big data and real-world data-based medicine in the management of hypertension. Hypertens Res 44, 147–153 (2021). https://doi.org/10.1038/s41440-020-00580-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-00580-3