Abstract
Individuals with metabolic syndrome reportedly have an increased risk of cardiovascular disease, although the association between asymptomatic myocardial damage and metabolic syndrome has not been sufficiently investigated. The present study investigated possible associations between circulating cardiac troponin and metabolic syndrome or related factors. Subjects undergoing their annual health checkups were enrolled in the study (n = 1242). Laboratory measurements included serum high-sensitivity cardiac troponin I (hs-cTnI) and plasma B-type natriuretic peptide (BNP). Individual salt intake was estimated by calculating 24-h urinary sodium excretion from spot urine. Subjects whose electrocardiograms revealed ST-T segment abnormalities or who had renal insufficiency or a history of cardiovascular events were excluded. Subjects with metabolic syndrome had higher hs-cTnI levels than those without, but their BNP levels were equivalent. hs-cTnI levels were significantly associated with the presence and components of metabolic syndrome. Logistic regression analysis with the endpoint of hs-cTnI levels higher than the median value identified metabolic syndrome as an independent determinant of increased hs-cTnI levels. Additionally, urinary salt excretion levels were increased in subjects with metabolic syndrome or any of its components. Logistic regression analysis with the endpoint of metabolic syndrome revealed that hs-cTnI levels were independently associated with the presence of metabolic syndrome. A close association between hs-cTnI levels and the presence of metabolic syndrome, at least partially mediated by increased salt intake, was confirmed to exist in the general population. The findings support the idea that patients with metabolic syndrome develop asymptomatic myocardial damage without obvious ischaemic findings, which leads to increased cardiovascular risk.
Similar content being viewed by others
Introduction
Metabolic syndrome is diagnosed based on body weight gain and accumulation of visceral fat accompanied by multiple cardiovascular risk factors [1,2,3]. The diagnostic criteria for metabolic syndrome comprise the following four components: visceral fat obesity indicated by elevated waist circumference, lipid metabolism disorder, elevated blood pressure (BP) and abnormal fasting glucose due to insulin resistance [4, 5]. Approximately 20–40% of adults in Western countries have been shown to have metabolic syndrome, although only ~10% of adults in Japan have been diagnosed with that condition [6,7,8,9]. The basic pathophysiology of metabolic syndrome is based on insulin resistance and inflammation that lead to sympathetic nerve activation, increased fluid retention and metabolic disorder and contribute to macrovascular and microvascular disease [10,11,12,13,14,15,16]. This may be one of the reasons why subjects with metabolic syndrome reportedly face an increased risk of cardiovascular disease, including coronary artery disease and heart failure, as well as all-cause mortality [9, 15,16,17,18].
Myocardial damage is apparent in patients with myocardial infarction, including acute coronary syndrome, and is often confirmed to be present in both acute and non-acute ischaemic myocardial disease [19, 20]. Alternatively, myocardial damage can progress asymptomatically through non-ischaemic mechanisms such as cardiac overload and inflammation [21, 22], with diagnosis based solely on circulating levels of constitutively expressed myocardial proteins such as the cardiac troponins detected incidentally in clinical settings [23, 24]. Elevated levels of cardiac troponins are also reported to occur with left ventricular overload caused by elevated BP [25, 26]. However, there are limited data on persistent myocardial damage in metabolic syndrome without obvious ischaemic conditions [27, 28]. We thus hypothesized that metabolic syndrome could be a cause of asymptomatic myocardial damage among patients in stable condition with no obvious ischaemic findings. To this end, the present study investigated possible associations between circulating high-sensitivity cardiac troponin I (hs-cTnI) and the presence of metabolic syndrome or related factors in individuals without obvious ischaemic findings on resting electrocardiograms.
Methods
The present study enrolled subjects attending their annual physical checkups for a study protocol approved by the ethics committees of Nagoya City University Graduate School of Medical Sciences and Enshu Hospital. The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to their participation in the study.
Subjects
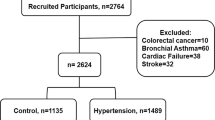
Subjects who visited Enshu Hospital in 2015–16 for an annual health checkup were screened for eligibility to participate in the present study. Subjects with renal dysfunction (creatinine ≥1.5 mg/dL), cancer, active inflammatory disease, or a history of cardiovascular events (stroke, myocardial infarction and heart failure) were excluded, as were subjects with obvious ST segment or T wave abnormality, implanted pacemakers or frequent arrhythmia (including atrial fibrillation and atrial flutter) on a standard 12-lead electrocardiogram. Since high-intensity physical activity can influence hs-cTnI levels, subjects who engaged in hard physical labour or high-intensity exercise were also excluded.
Participants were instructed to collect overnight urine in a paper cup and to submit a sample of the urine in a plastic tube. Salt intake was assessed by estimating 24-h urinary sodium excretion, calculated using the Kamata formula [29], which estimates 24-h sodium excretion based on lean body mass and the composition of overnight urine in the Japanese population, achieving a correlation coefficient of 0.78 between measured and estimated sodium excretion [29]. Then, blood samples were taken early in the morning after an overnight fast for laboratory measurements, including serum concentrations of hs-cTnI. Systolic and diastolic BPs were measured in the non-dominant arm of seated subjects using a validated oscillometric technique (HEM-7070; Omron Corporation, Kyoto, Japan). Three consecutive BP measurements were taken at 2-min intervals, and the mean of the second and third measurements was recorded as the BP. Subjects taking antihypertensive medications or having systolic BP ≥140 mmHg and diastolic BP ≥90 mmHg were defined as having hypertension [30]. Subjects taking lipid-lowering medications or having high-density lipoprotein cholesterol (HDL-C) levels <40 mg/dL, low-density lipoprotein cholesterol (LDL-C) levels ≥140 mg/dL or triglycerides ≥150 mg/dL were defined as having dyslipidaemia [31]. Subjects taking antihyperglycaemic medication or presenting a fasting plasma glucose (FPG) level ≥126 mg/dL were defined as having diabetes [32]. Metabolic syndrome was defined based on the Japanese diagnostic criteria (abdominal obesity, with waist circumference ≥85 cm for men or ≥90 cm for women, and two or more of the following three criteria: (1) triglycerides ≥150 mg/dL and/or HDL-C <40 mg/dL; (2) systolic BP ≥130 mmHg and/or diastolic BP ≥85 mmHg; and (3) FPG ≥110 mg/dL) [4].
Biochemical analysis
Biochemical tests including determination of serum total cholesterol, LDL-C, HDL-C, and triglycerides were performed using standard laboratory assays. Plasma B-type natriuretic peptide (BNP) concentrations were determined using a commercially available chemiluminescence enzyme immunoassay (MI02 Shionogi BNP Kit; Shionogi, Osaka, Japan). Circulating levels of hs-cTnI were measured by the ARCHITECT high-sensitive troponin I assay according to the manufacturer’s instructions (Abbott, Tokyo, Japan).
Statistical analysis
Data were analysed using IBM SPSS Statistics 19 (IBM Corp., Chicago, IL, USA). Dichotomous variables (gender, smoking status and presence or absence of metabolic syndrome) were assigned values of 0 (female, non-smoker and absence of metabolic syndrome) or 1 (male, smoker and presence of metabolic syndrome). Data with a normal distribution are expressed as the mean ± standard deviation. Data that are not normally distributed (BNP and hs-cTnI) are expressed as the median with interquartile range and are evaluated after log transformation. Comparative analyses of continuous variables were performed using t tests. Differences in continuous variables among more than three groups were tested using analysis of variance followed by a post hoc Scheffe test. Logistic regression analysis determined the independent variables associated with increased hs-cTnI and the presence of metabolic syndrome. Receiver operating characteristic (ROC) curve analyses were used to select a cut-off level for hs-cTnI. The median value of hs-cTnI was used as the cut-off value defining increased hs-cTnI. A two-tailed P < 0.05 value was considered significant.
Results
Of the 1242 subjects enrolled in the study, the numbers (percentages of the total) with hypertension, dyslipidaemia, diabetes mellitus and obesity (body mass index >25 kg/m2) were 390 (31.4%), 617 (49.7%), 95 (7.6%) and 238 (19.2%), respectively. In addition, 148 subjects (11.9%), comprising 115 (9.3%) males and 33 (2.7%) females, fulfilled the diagnostic criteria for metabolic syndrome (Table 1).
Subjects with metabolic syndrome had higher levels of hs-cTnI than those without, while BNP levels were equivalent in those with and without metabolic syndrome (Table 1). Similarly, subjects with any given component of metabolic syndrome or with at least one of the components of metabolic syndrome and abdominal obesity (the preliminary conditions for metabolic syndrome) had significantly higher levels of hs-cTnI than those without (Figs. 1 and 2a), and subjects with the preliminary conditions for metabolic syndrome had higher levels of hs-cTnI than normal subjects even after the exclusion of hypertensive subjects (Fig. 2b). Logistic regression analysis examined how the presence of metabolic syndrome affected the elevation of hs-cTnI, with the endpoint of hs-cTnI levels higher than the median value. The analysis showed that the presence of metabolic syndrome was an independent determinant of increased hs-cTnI levels after adjustment for age and gender (Table 2, adjusted Model 1) and further adjustment for smoking status and creatinine (Table 2, adjusted Model 2). Additional analysis showed that the presence of metabolic syndrome was significantly associated with increased hs-cTnI levels after adjustment for medications (Table 2, adjusted Model 3).
Log-transformed circulating high-sensitivity cardiac troponin I (hs-cTnI) levels in subjects with and without the following components of metabolic syndrome: a abdominal obesity (waist circumference ≥85 cm for men and ≥90 cm for women), b lipid metabolism disorder (triglycerides ≥150 mg/dL and/or high-density lipoprotein cholesterol <40 mg/dL), c elevated blood pressure (systolic pressure ≥130 mmHg and/or diastolic pressure ≥85 mmHg), and d abnormal fasting plasma glucose (fasting plasma glucose ≥110 mg/dL). Data are shown as the mean ± standard deviation
Log-transformed circulating high-sensitivity cardiac troponin I (hs-cTnI) levels in normal subjects without any of the components of metabolic syndrome (Group A), subjects with at least one of the components of metabolic syndrome excluding abdominal obesity (Group B), subjects with at least one of the components of metabolic syndrome including abdominal obesity (Group C), and subjects with metabolic syndrome (Group D), assessed a regardless of blood pressure and b in subjects without hypertension. Log-transformed urinary salt excretion levels in Group A, Group B, Group C and Group D assessed c regardless of blood pressure and d in subjects without hypertension. Data are shown as the mean ± standard deviation. *P < 0.0001 vs. normal subjects (Group A)
We then examined the effects of urinary salt excretion levels on metabolic syndrome based on the reported association of salt intake with insulin resistance and fluid retention. The analysis showed that urinary salt excretion levels were higher in subjects with metabolic syndrome or the preliminary conditions for metabolic syndrome than in normal subjects (Fig. 2c), even after excluding subjects with hypertension from the analysis (Fig. 2d). ROC curve analysis yielded 2.6 pg/ml as the cut-off hs-cTnI level indicating metabolic syndrome (area under the curve 0.658, P < 0.01, 95% confidence interval 0.630–0.684), sensitivity 66.9% (95% confidence interval 58.7–74.4), specificity 59.0% (95% confidence interval: 56.0–61.9) (Supplementary Figure). Logistic regression analysis with the presence of metabolic syndrome as the endpoint showed that hs-cTnI levels were independently associated with metabolic syndrome after adjustment for age and gender (Table 3, adjusted Model 1) and further adjustment for smoking status and creatinine (Table 3, adjusted Model 2). Urinary salt excretion was added to the analysis in adjusted Model 2 and was also identified as an independent determinant of metabolic syndrome (Table 3, adjusted Model 3).
Discussion
The main findings of the present study are as follows: (i) subjects with metabolic syndrome had higher levels of hs-cTnI, but not BNP, than those without; (ii) subjects with any given component of metabolic syndrome or at least one component of metabolic syndrome showed significantly higher levels of hs-cTnI and urinary salt excretion than those without; (iii) logistic regression analysis with the endpoint of hs-cTnI levels higher than the median value showed that the presence of metabolic syndrome was an independent determinant of increased hs-cTnI; and (iv) logistic regression analysis with the endpoint of meeting the diagnostic criteria for metabolic syndrome showed that hs-cTnI was independently associated with the presence of metabolic syndrome after adjustment for possible confounding factors including urinary salt excretion. These results indicate that the presence of metabolic syndrome is significantly associated with asymptomatic myocardial damage through increased salt intake.
Metabolic syndrome is associated with high risks of cardiovascular disease and mortality [14,15,16,17,18], and although the underlying mechanism is not fully understood, the increased incidence of macrovascular disease, mainly coronary artery disease, is thought to be related [14]. Since insulin resistance and vascular inflammation promote atherosclerotic cardiovascular disease, metabolic syndrome is associated with the progression of atherosclerotic plaque and instability of the plaque, leading to obstructive coronary artery disease and acute coronary syndrome [14,15,16,17,18]. Although significant associations between cardiac troponin and metabolic syndrome were reported previously, subjects with ST-T segment abnormalities in the electrocardiogram had not been excluded from the previous studies; therefore, subjects with coronary artery disease may have been included [27, 28]. In contrast, microvascular disease relevant to metabolic syndrome could be considered a small vessel disorder caused by microvascular endothelial dysfunction [11,12,13,14]. Such microvascular disease could develop silently in metabolic syndrome without increasing cardiac load and could thus cause asymptomatic cardiovascular disease. The independent association of hs-cTnI, but not BNP, with the presence of metabolic syndrome as demonstrated in the present study may be caused by microvascular disorder and suggests that subjects with metabolic syndrome should be treated carefully even in the absence of elevated BNP levels. In addition, if possible, hs-cTnI levels should be assessed repeatedly in individuals with metabolic syndrome. On the other hand, the median level of hs-cTnI was significantly higher in subjects with metabolic syndrome than in those without (3.4 vs. 2.3 pg/ml). The cut-off level of hs-cTnI (2.6 pg/ml) suggested by ROC curve analysis as a threshold for metabolic syndrome was only slightly higher than the median value in subjects without metabolic syndrome but was not sufficiently robust as a diagnostic indicator for single tests in a clinical setting. These results also suggest that attention should be paid to subjects with obesity or cardiovascular risk factors and hs-cTnI levels higher than the cut-off level, even in the absence of metabolic syndrome.
We previously found that left ventricular voltage measured by electrocardiography was independently associated with hs-cTnI levels even after adjustment for BNP [33]. In the present study, hs-cTnI levels were increased in subjects with at least one of the components of metabolic syndrome even when hypertensive subjects were excluded from the analysis. Moreover, the presence of metabolic syndrome was an independent determinant of increased hs-cTnI levels after adjustment for systolic and diastolic BP. Additionally, to adjust for possible confounding factors including all components of metabolic syndrome, we conducted further logistic regression analysis with the endpoint of hs-cTnI levels higher than the median value and found that the presence of metabolic syndrome was an independent determinant of high hs-cTnI (Supplementary Table 1). Moreover, similar logistic regression analysis with the endpoint of metabolic syndrome showed that hs-cTnI was independently associated with the presence of metabolic syndrome (Supplementary Table 2). Thus, metabolic syndrome significantly affects hs-cTnI levels independently of hypertension. These findings support the hypothesis that both cardiac load and metabolic disorder are causally associated with asymptomatic myocardial damage. Indeed, insulin resistance and metabolic disorder have been implicated in cardiovascular disease through the mechanisms of inflammation, oxidative stress, fibrosis and alteration in cardiac function [34,35,36,37]. Although an association between metabolic syndrome and systemic inflammation has been reported previously, the association between myocardial damage and inflammation during metabolic syndrome remains to be intensively investigated [38, 39]. Evaluating the relationship between hs-cTnI and high-sensitivity C-reactive protein was beyond the scope of the present study; however, we speculate that systemic inflammation is one of the pathological pathways leading to myocardial injury in metabolic syndrome. Previous animal studies have implied that obesity and insulin resistance cause mitochondrial dysfunction and an increase in fatty acids, leading to increased oxidative stress, release of inflammatory cytokines such as tumour necrosis factor-α and interleukin-6, and a change in the balance of adipokines [40,41,42]. These disorders affecting the heart could contribute to asymptomatic myocardial damage in metabolic syndrome. On the other hand, urinary salt excretion levels were assessed with respect to metabolic syndrome in the present study, since obesity and insulin resistance might be associated pathophysiologically [13,14,15,16]. As expected, urinary salt excretion levels were increased in subjects with metabolic syndrome or its components even if hypertensive subjects were excluded, indicating that an increase in salt intake is an independent determinant of the presence of metabolic syndrome. Importantly, these results are in line with previous reports that sodium intake affects all components of metabolic syndrome [15, 16]. Moreover, sodium restriction was reported to reduce cardiac injury by reducing inflammation and oxidative stress in a rat model of metabolic syndrome [43, 44]. Therefore, metabolic syndrome might occur through a pathway relevant to sodium and volume retention through insulin resistance and inflammation, leading to asymptomatic myocardial damage. Conversely, hs-cTnI was an independent determinant of metabolic syndrome after adjustment for possible confounding factors. These findings emphasize the strong association between metabolic syndrome and asymptomatic myocardial damage.
Cardiac troponin levels are the mainstay of diagnosing acute coronary syndrome [18, 19] but are also used for differentiating myocardial damage from coronary artery disease [22, 45]. Indeed, the usefulness of cardiac troponins as predictors of cardiovascular events in non-ischaemic heart disease has been demonstrated [46, 47]. Thus, since a mild increase in cardiac troponins even within the normal range has been associated with future cardiovascular events, assessment of myocardial damage is meaningful in the general population, whose cardiac troponin levels would not be high [48]. Cardiac troponin is a complex of three types of regulatory proteins, called troponin T, troponin I, and troponin C. Cardiac troponin T and I are often used as cardiac biomarkers, whereas cardiac troponin C has complete amino acid homology with skeletal muscle troponin C and thus is not used as a cardiac biomarker [49]. In addition, cardiac troponin T levels can be elevated in patients with reduced renal function, and cardiac troponin I is superior to troponin T in the detection of electrocardiographic cardiac injury [50]. Hence, we focused on troponin I to detect myocardial damage when investigating the relationship between hs-cTnI and metabolic syndrome. Therefore, findings obtained from the present study suggest that detecting patients with both metabolic syndrome and increased hs-cTnI levels is meaningful for identifying individuals with high cardiovascular risk and mortality.
The present study has several limitations, and the findings should thus be interpreted with caution. First, the present study was a cross-sectional study, and the background of the subjects enrolled in the study was heterogeneous. Second, the causal relationship between hs-cTnI levels and metabolic syndrome was not investigated, and biological assessments to demonstrate the mechanisms underlying the close association between hs-cTnI levels and metabolic syndrome in vivo are needed in the future. Third, enrolled subjects were asymptomatic and without ST-T abnormalities on their electrocardiograms, but neither the exercise stress test nor coronary angiography was performed to exclude ischaemic heart disease. Fourth, other variables that might affect metabolic syndrome or hs-cTnI, such as diet, physical activity, sleep, and genetic variance, were not taken into account. Further investigations with a larger population, a longitudinal design and detailed examination are also necessary for definite conclusions to be drawn.
In conclusion, a close association between circulating hs-cTnI levels and the presence of metabolic syndrome, at least partially mediated by increased salt intake, in the general population without electrocardiographic ST-T segment abnormalities. The significant association between asymptomatic myocardial damage and metabolic syndrome might be causally involved in high-risk cardiovascular disease and mortality in metabolic syndrome without obvious ischaemic findings.
References
Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7.
Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120:S3–S8.
Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49.
Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Definition and the diagnostic standard for metabolic syndrome—Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Nihon Naika Gakkai Zasshi. 2005;94:794–809.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24.
Noda H, Iso H, Saito I, Konishi M, Inoue M, Tsugane S, JPHC Study Group. The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: the Japan public health center-based study. Hypertens Res. 2009;32:289–98.
Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120:S10–S16.
Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf). 2009;196:193–222.
Czernichow S, Greenfield JR, Galan P, Jellouli F, Safar ME, Blacher J, et al. Macrovascular and microvascular dysfunction in the metabolic syndrome. Hypertens Res. 2010;33:293–7.
Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20:140–6.
Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43.
Oh SW, Han KH, Han SY, Koo HS, Kim S, Chin HJ. Association of sodium excretion with metabolic syndrome, insulin resistance, and body fat. Medicine (Baltim). 2015;94:e1650.
Won JC, Hong JW, Noh JH, Kim DJ. Association between estimated 24-h urinary sodium excretion and metabolic syndrome in Korean Adults: the 2009 to 2011 Korea National Health and Nutrition Examination Survey. Medicine (Baltim). 2016;95:e3153.
Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: a meta-analysis of prospective cohort studies. Medicine (Baltim). 2017;96:e8491.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32.
Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol. 2001;38:478–85.
Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67.
deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502.
Guzzardi MA, Iozzo P. Fatty heart, cardiac damage, and inflammation. Rev Diabet Stud. 2011;8:403–17.
Reiter M, Twerenbold R, Reichlin T, Benz B, Haaf P, Meissner J, et al. Early diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assays. Eur Heart J. 2012;33:988–97.
Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JAC, et al. High-sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation. 2017;135:1494–505.
Aeschbacher S, Schoen T, Bossard M, van der Lely S, Glättli K, Todd J, et al. Relationship between high-sensitivity cardiac troponin I and blood pressure among young and healthy adults. Am J Hypertens. 2015;28:789–96.
Sugiura T, Dohi Y, Takase H, Ito A, Fujii S, Ohte N. Differential effects of brachial and central blood pressures on circulating levels of high-sensitivity cardiac troponin I in the general population. Atherosclerosis. 2018;269:185–91.
Milwidsky A, Fisher E, Brzezinski RY, Ehrenwald M, Shefer G, Stern N, et al. Metabolic syndrome is associated to high-sensitivity cardiac troponin T elevation. Biomarkers. https://doi.org/10.1080/1354750X.2018.1528630.
Pokharel Y, Sun W, Villareal DT, Selvin E, Virani SS, Ndumele CE, et al. Association between high-sensitivity troponin T and cardiovascular risk in individuals with and without metabolic syndrome: The ARIC study. Eur J Prev Cardiol. 2017;24:628–38.
Kamata K, Tochikubo O. Estimation of 24-h urinary sodium excretion using lean body mass and overnight urine collected by a pipe-sampling method. J Hypertens. 2002;20:2191–7.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–392.
Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:155–8.
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–28.
Sugiura T, Dohi Y, Takase H, Fujii S, Ohte N. Findings relevant to the QRS wave in the resting electrocardiogram are associated with circulating concentrations of high-sensitivity cardiac troponin I in the general population. J Am Soc Hypertens. 2018;12:614–20.
Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102.
Cauwenberghs N, Knez J, Thijs L, Haddad F, Vanassche T, Yang WY, et al. Relation of insulin resistance to longitudinal changes in left ventricular structure and function in a general population. J Am Heart Assoc. 2018;7:e008315.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122.
Wu C, Liu K, Bertoni AG, Ouyang P, Bluemke DA, Lima JA. Metabolic syndrome is associated with impaired diastolic function independently of MRI-derived myocardial extracellular volume: the MESA study. Diabetes. 2018;67:1007–12.
Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–54.
Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol. 2007;49:1798–805.
Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–95.
Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–47.
Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068–76.
Hattori T, Murase T, Takatsu M, Nagasawa K, Matsuura N, Watanabe S, et al. Dietary salt restriction improves cardiac and adipose tissue pathology independently of obesity in a rat model of metabolic syndrome. J Am Heart Assoc. 2014;3:e001312.
Jover B, Reynes C, Rugale C, Reboul C, Jeanson L, Tournier M, et al. Sodium restriction modulates innate immunity and prevents cardiac remodeling in a rat model of metabolic syndrome. Biochim Biophys Acta. 2017;1863:1568–74.
Aeschbacher S, Schoen T, Bossard M, van der Lely S, Glättli K, Todd J, et al. Relationship between high-sensitivity cardiac troponin I and blood pressure among young and healthy adults. Am J Hypertens. 2015;28:789–96.
Sugiura T, Takase H, Toriyama T, Goto T, Ueda R, Dohi Y. Circulating levels of myocardial proteins predict future deterioration of congestive heart failure. J Card Fail. 2005;11:504–9.
van der Linden N, Klinkenberg LJ, Bekers O, Loon LJ, Dieijen-Visser MP, Zeegers MP, et al. Prognostic value of basal high-sensitive cardiac troponin levels on mortality in the general population: a meta-analysis. Medicine (Baltim). 2016;95:e5703.
Willeit P, Welsh P, Evans JDW, Tschiderer L, Boachie C, Jukema JW, et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J Am Coll Cardiol. 2017;70:558–68.
Gomes AV, Potter SD, Szczesna-Cordary D. The role of troponin in muscle contraction. Life. 2002;54:323–33.
Kimenai DM, Martens RJH, Kooman JP, Stehouwer CDA, Tan FES, Schaper NC, et al. Troponin I and T in relation to cardiac injury detected with electrocardiography in a population-based cohort—the Maastricht study. Sci Rep. 2017;7:6610.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sugiura, T., Dohi, Y., Takase, H. et al. Close association between circulating high-sensitivity cardiac troponin I and metabolic syndrome in the general population. Hypertens Res 42, 1768–1775 (2019). https://doi.org/10.1038/s41440-019-0283-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0283-x
Keywords
This article is cited by
-
Elevated circulating high-sensitivity cardiac troponin t and cardiac remodeling in obesity
BMC Cardiovascular Disorders (2021)
-
Circulating Cardiac Biomarkers in Diabetes Mellitus: A New Dawn for Risk Stratification—A Narrative Review
Diabetes Therapy (2020)