Abstract
Binge drinking (BD) during adolescence is related to hypertension. There are, however, few studies concerning the effects of BD on kidney function and osmotic balance in relation to arterial pressure. The mechanism by which BD affects kidney function is related to oxidation and inflammation. Recently, Se, an essential trace element possessing antioxidant properties, has also been shown to be related to renal Na+/K+-ATPase activity. This study examined the protective effects of 0.4 ppm selenite administered to adolescent rats in an intermittent i.p. BD model. BD consumption depleted kidney and serum Se deposits, decreased GPx activity, and increased biomolecule oxidation in these locations. In the kidneys, GPx1, GPx3, GPx4, and NF-κB expression also decreased, coinciding with an increase in caspase-3 expression. BD decreased creatinine clearance and fractional Na+ excretion (EFNa), increased transtubular K+ excretion (TTKG) and serum aldosterone (Aldo) levels, and reduced relative Aldo clearance. These effects led to hypernatremia, low urinary flow, and high systolic blood pressure. Se supplementation to BD rats significantly improved oxidative balance, and kidney GPx, NF-κB, and caspase-3 expression; slightly increased EFNa and slightly decreased TTKG and serum Aldo levels; and greatly increased relative Aldo clearance. Se supplementation did not, however, modify creatinine clearance. In conclusion, BD triggers kidney osmotic and ionic imbalances, which contribute to increasing systolic blood pressure. These disturbances could be related in part to Se and selenoprotein GPxs, which decrease oxidative, inflammatory and apoptotic alterations in the kidneys. Se supplementation prevents these changes, improves ionic disturbances, and decreases serum Aldo levels and systolic blood pressure.
Similar content being viewed by others
Introduction
From both a clinical and a public point of view, intermittent binge drinking (BD) during adolescence is the most important alcohol consumption pattern [1]. The effects of BD intoxication have been associated with an elevated risk of hypertension (HTN), yet all of the mechanisms involved have not been well elucidated [2].
HTN is closely related to renal dysfunction. The associated mechanism involves the dysregulation of renal sodium and water excretion, which is mainly related to a shift in renal pressure natriuresis and/or an increase in antinatriuretic hormones such as aldosterone (Aldo) and angiotensin II [3]. These hormones also reduce renal excretory capability, decrease glomerular filtration rate (GFR), and constrict the peripheral vasculature. Recently, the hormones have also been found to be related to the genesis of vascular oxidative stress [4], initiating a sequence of events that elevate blood pressure.
Ethanol has deleterious structural and functional effects on the kidneys [5, 6]. Large amounts of consumed ethanol, such as those in a BD ethanol consumption model, are metabolized in the kidneys by the enzyme alcohol dehydrogenase and by the microsomal ethanol-oxidizing system, both of which are related to the generation of pro-oxidative reactive oxygen species (ROS) [7,8,9]. Since renal lipids are rich in long-chain polyunsaturated fatty acids [10]; the kidneys are highly vulnerable to ROS-induced damage.
At present, oxidative stress is considered to be the primary route to ethanol-induced kidney injury, since ethanol increases lipid oxidation by increasing ROS production and decreasing the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) [7]. Lipid oxidation of nephron epithelial cells leads to changes in membrane composition, interfering with carrier functions such as Na+/K+-ATPase activity, which is enhanced after chronic alcohol exposure, and increasing sodium reabsorption and potassium excretion [8].
After acute ethanol administration, disturbances in renal electrolyte excretion [11] and decreases in GFR have also been described, implying increases in water retention [12]. High systemic ROS production is one of the mechanisms by which acute ethanol [13] stimulates the sympathetic nervous system (SNS), the hypothalamus–hypophysis–adrenal axis (HHA), and the renin-angiotensin-aldosterone system (RAAS), contributing to increased concentrations of antinatriuretic hormones and renal vasoconstriction, and leading to HTN [2].
Selenium (Se), an essential trace element, is the catalytic center of 25 different selenoproteins, such as the antioxidant proteins in the GPx family and the plasma Se transporter selenoprotein P (SelP), which also has antioxidant properties [14]. Ojeda et al. [15] found that, during adolescence, BD alters Se homeostasis and Se tissue distribution, and that dietary Se supplementation is a good strategy against BD liver damage, since Se decreases oxidative hepatic damage by increasing GPx1 and GPx4 hepatic expression, thus preventing NF-κB downregulation and apoptosis [16].
The most important selenoprotein produced in rodent kidney tissues is GPx3 [17]. GPx3 acts in plasma, reducing hydrogen peroxide to water, but it also acts in the proximal tubules of nephrons [18]. GPx4 is the second main selenoprotein synthesized in the kidneys [19]. It is the only GPx member that reduces hydroperoxides in lipoproteins, complex lipids, and phospholipids of biomembranes, and it plays an essential antioxidant role in mitochondria, modulating the intrinsic apoptotic pathway, and activating the transcription factor NF-κB [20]. SelP is also synthesized in the kidneys at even higher levels than GPx1 and has antioxidant cytosolic actions [19]. Importantly, Ojeda et al. [15] found Se depletion in the kidneys of adolescent BD rats. A previous study [21] also found that Se supplementation to chronic ethanol-exposed pups improved kidney Se deposits and renal development and decreased renal oxidation by increasing GPx activity but did not improve GFR. Ozkol et al. [22] described the protective effects of Se supplementation against acute ethanol intoxication in rat kidneys, which were mediated by the antioxidant capacity of Se.
Given all of these findings, the main objective of this study was to evaluate kidney selenoprotein balance in adolescent BD-exposed rats and its relationship with oxidative balance, apoptotic and inflammatory status, and kidney function in relation to blood pressure control (through analysis of GFR, electrolytes, and water reabsorption). To determine the potential of Se as a therapy against kidney damage and HTN in adolescent BD consumers, the same parameters were analyzed after Se supplementation in this animal model.
Methods
Animals
Thirty-two adolescent male Wistar rats (Centre of Production and Animal Experimentation, Vice-Rector’s Office for Scientific Research, University of Seville) were used in these experiments. The rats were received when they were 21 days old and were housed in groups of two rats per cage for 1 week to acclimatize to the housing conditions and handling. The experimental treatment was conducted over a 3-week period beginning when the rats reached postnatal day (PND) 28 and ending at 47 days of age. This period corresponds to adolescence in Wistar rats [23]. The animals were kept at an automatically controlled temperature (22–23 °C) under a 12-h light/dark cycle (light: 09:00–21:00). The animal care procedures and experimental protocols were performed in accordance with EU regulations (Council Directive 86/609/EEC, 24 November 1986) and were approved by the Ethics Committee of the University of Seville.
On PND 28, when the adolescent period began, the rats were randomly assigned into four groups (n = 8/group) according to their treatments. The control (C) group rats were given a control diet and drinking water ad libitum and were given an isotonic saline solution (SSF) intraperitoneally (i.p.) on the indicated days. The BD group rats were given a control diet and drinking water ad libitum and were given an ethanol solution (20% v/v) in isotonic saline (3 g/kg/d) i.p. on the indicated days. The control selenium-supplemented (CSe) group rats were given a control diet and Se-supplemented drinking water ad libitum and injected with SSF on the indicated days. The binge-drinking selenium-supplemented (BDSe) group rats were given a control diet and drinking water supplemented with Se ad libitum and were given an alcohol solution (20% v/v) in isotonic saline (3 g/kg/d) i.p. on the indicated days.
A standard pellet diet (2014 Teklad Global 14% Protein Rodent Maintenance Diet, Harlan Laboratories, Barcelona, Spain) that contained 0.23 ppm Se was available ad libitum to all the experimental groups. The Se-supplemented groups (CSe and BDSe) received 0.14 ppm Se extra as anhydrous sodium selenite (Panreac, Barcelona, Spain) in drinking water during all experimental periods.
Nutritional control
Body weight and the amount of food consumed by the rats were monitored daily until the end of the experimental period. The amounts of food and drinking water ingested every day were calculated; the amounts of food and water were measured every morning, and the differences in the amounts between consecutive days were defined as the amounts consumed. Se intake was calculated by multiplying the known Se concentration (ppm) in the diet and the drinking water by the amounts of food and water ingested every day. All measurements were taken at 9:00 a.m. to avoid changes due to circadian rhythms.
Ethanol treatment
The alcohol-exposed groups (BD and BDSe) received i.p. injections of alcohol (20% v/v) in SSF (3 g/kg/d). This animal model is one of the most commonly used to reproduce repeated BD, since it easily ensures a blood alcohol concentration of 80 mg/dL (the value established by the NIAAA to reflect BD) [24]. The alcohol injections were given starting at 7:00 p.m., when the dark cycle began, for 3 consecutive days each week for 3 weeks. No i.p. injections were given during the remaining 4 days of each week [25]. The control groups (C and CSe) received i.p. injections of equal volumes of SSF at the same time as when the alcohol-exposed groups received alcohol injections.
Blood pressure (mmHg) and heart rate (beats/min)
Systolic blood pressure (SBP) was monitored with a pressure meter (NIPREM 645, CIBERTEC, Madrid, Spain) using the indirect tail occlusion method. Measurements were taken in adolescent rats 24 h after the last alcohol exposure or the last injection with SSF. The signals were collected via a data acquisition system coupled to the pressure meter. The blood pressure of each animal was measured times successively to calculate the arithmetic mean, which was the value used.
Samples
At the end of the experimental period, the rats were fasted for 12 h, and feces and urine samples were collected using individual metabolic cages. Then, 24 h after the last alcohol exposure or treatment with SSF, the adolescent rats were anesthetized with an i.p. injection of 28% w/v urethane (0.5 ml/100 g of body weight). Blood was obtained by heart puncture and collected in tubes. Serum was prepared using low-speed centrifugation for 15 min at 1300 × g. The abdomen was opened with a midline incision, and the whole kidneys were removed, debrided of adipose tissue, weighed, frozen in liquid nitrogen and stored at −80 °C prior to biochemical determinations.
Selenium analysis
Serum, urine, and kidney Se levels were determined by graphite furnace atomic absorption spectrometry. We used a PerkinElmer AAnalyst 800 high-performance atomic absorption spectrometer with WinLab32 for AA software equipped with a transversely heated graphite furnace, a longitudinal Zeeman-effect background corrector, and an AS furnace autosampler (PerkinElmer, Ueberlingen, Germany). The sources of radiation were electrodeless Se discharge lamps. The instrumental operating conditions and the reagents were the same as those described by Ojeda et al. [26]. After 72 h at a dry temperature of 100 °C, the kidney samples were digested in a sand bath heater (Ovan) for 72 h with nitric acid, and perchloric acid and chloridric acid (6 N) were added. The serum samples were diluted fivefold in 0.2% v/v HNO3 and 0.2% Triton X-100 solutions, and the urine samples were diluted 1:2 v/v.
Antioxidant enzymes and oxidative stress markers
To measure the activity of antioxidant enzymes (SOD, CAT, GPx, and GR), as well as the levels of lipid and protein oxidation (as indicated by malondialdehyde and carbonyl group levels, respectively), kidney tissue samples were homogenized (100 × g for 1 min, 1:4 w/v) using a Potter homogenizer (Pobel 245432, Madrid, Spain) in sucrose buffer (15 mM Tris-HCl, pH 7.4, 250 mM sucrose, 1 mM EDTA and 1 mM dithiothreitol) in an ice bath. The homogenate was centrifuged at 900 × g for 10 min at 4 °C. The resulting supernatant was used for biochemical assays according to techniques described by Ojeda et al. [21]. GPx activity and lipid and protein oxidation were also determined in serum samples.
Immunoblotting assays
The expression of the selenoproteins GPx1, GPx3, GPx4, and SelP as well as NF-κB p65 and caspase-3 was determined in the kidneys of adolescent rats. The samples utilized contained 200 µg of protein. The proteins were separated on polyacrylamide gels and transferred to a nitrocellulose membrane (Immobilon-P Transfer Membranes, Millipore, Billerica, MA, USA) using a blot system (Transblot, Bio-Rad, CA, USA). Nonspecific membrane sites were blocked for 1 h with a blocking buffer containing TTBS (50 mM Tris-HCl, 150 mM NaCl, 0.1% (v/v) Tween 20, pH 7.5) and 3% milk powder (Bio-Rad); thereafter, the membrane was probed overnight at 4 °C with specific primary antibodies (rabbit polyclonal IgG, Santa Cruz Biotechnology) against the following proteins: GPx1 (1:2000 dilution), GPx3 (1:2000 dilution), GPx4 (1:5000 dilution), SelP (1:2500 dilution), NF κB p-65, and cleaved caspase-3 (1:1000 dilution). Secondary antibodies (anti-rabbit IgG HRP conjugate, Santa Cruz Biotechnology) were utilized at the following dilutions: 1:5000 for GPx1 and GPx3, 1:10,000 for GPx4, 1:5000 for SelP, 1:2500 for NF κB p-65, and caspase-3. Monoclonal mouse anti-β-actin (IgG1 A5441, Sigma-Aldrich, Madrid, Spain) was used to detect β-actin as a loading control at a dilution of 1:20,000, and a secondary antibody, anti-mouse IgG peroxidase conjugate (A9044, Sigma-Aldrich), was used at a dilution of 1:8000. The membrane was incubated for 1 min with a commercial developing solution, luminol ECL reagent (GE Healthcare and Lumigen, Buckinghamshire, UK). The quantification of the blots was performed by densitometry with PCBAS 2.08e analysis software (Raytest, Straubenhardt, Germany). The results are expressed as percent arbitrary units relative to the values in control animals, which were defined as 100%.
Clearance measurement
The creatinine, albumin, urea, Na+, and K+ levels in serum and urine were determined at Valme University Hospital by routine clinical biochemistry tests with a Cobas 6000 module (Roche Diagnostics GmbH), according to the manufacturer’s specifications and using proprietary reagents. The instrument was calibrated against appropriate proprietary reference standard material and verified with the use of proprietary quality controls. Aldo levels in both serum and urine were determined using an Aldosterone ELISA Kit (Enzo Life Sciences, Switzerland) based on the binding of Aldo to a specific antibody that was immobilized on the walls of a 96-well plate. Urine and plasma osmolality were determined by the vapor pressure technique in a model 5100C osmometer (Wescor, USA).
Na+, K+, Se, urea, Aldo, and creatinine clearances were calculated from the standard formula clearance (CL) = U × V/P, where U is the level of the substance to be cleared in urine, V is the volume of urine collected in 24 h, and P is the level of the substance in plasma. The relative clearances of Na+, K+, Se, urea, and Aldo were calculated as CLx/CL creatinine × 100, where x is the substance to be compared. The fractional excretion of sodium (FENa) and the transtubular potassium gradient (TTKG) were calculated by the standard formulae FENa = UNa+ × PCr/PNa+ × UCr and TTKG = (POsm × UK+)/(PK+ × UOsm), where U is the level of the substance in urine, P is the level of the substance in plasma, and Osm is the osmolality.
Statistical analysis
The results are expressed as the mean ± standard error of the mean. The data were analyzed using a statistical program (GraphPad InStat 3, CA, USA) by analysis of variance (one-way ANOVA). Statistical significance was established at p < 0.05. When ANOVA resulted in differences, multiple comparisons between means were conducted with the Tukey–Kramer test.
Results
Intermittent i.p. exposure to BD did not alter total kcal intake or weight gain (Table 1). However, it significantly altered liquid homeostasis, since BD rats consumed less water (p < 0.05) and had extremely significantly lower urinary flow (p < 0.001) than control rats. Se supplementation to BD rats prevented the reduction in water intake but did not change the low urinary flow. The relative kidney weight and protein content were similar among the four experimental groups. However, Se kidney deposits were significantly lower in BD rats than in C rats (p < 0.01). Se supplementation significantly increased Se kidney deposits in control and ethanol-exposed rats.
In the kidneys, BD exposure significantly increased SOD, CAT, and GR antioxidant activity and decreased GPx activity. Protein and lipid oxidation did, however, take place (Fig. 1). Se supplementation to these animals mainly increased the activity of the selenoprotein GPx and decreased the activity of GR, modulating the activity of this pair of enzymes and decreasing kidney lipid oxidation. In Se-supplemented control rats, an increase in SOD and GPx activity was observed that did not affect biomolecule oxidation.
Oxidative balance in the kidneys: SOD activity (a), CAT activity (b), GPx activity (c), GR activity (d), lipid (e), and protein (f) oxidation. The results are expressed as the mean ± SEM and were analyzed by multifactorial analysis of variance (one-way ANOVA) followed by Tukey’s test. The number of animals in each group was 8. Groups: C, control group; BD, binge-drinking group; CSe, control selenium-supplemented group; BDSe, binge-drinking selenium-supplemented group. Significant differences between groups are expressed as p values and are indicated by the asterisk for comparisons between the C and BD groups, by the superscript ‘a' for comparisons between the BD and BDSe groups, by the superscript ‘c' for comparisons between the C and CSe groups, and by the superscripted solid circle for comparisons between the CSe and BDSe groups
Binge ethanol consumption decreased the expression of the selenoproteins GPx1, GPx3, and GPx4 (Fig. 2). Consistent with the reduction in the antioxidant protein expression found, NF-κB levels also decreased and caspase-3 levels increased in BD rats. Se supplementation prevented these effects by increasing GPx and NF-κB expression and reducing caspase-3 expression. Se supplementation to control rats significantly increased GPx1, GPx3, and SelP expression compared to no supplementation, yet it did not modify NF-κB and caspase-3 expression.
Expression of selenoproteins (a GPx1; b GPx3; c GPx4; and d SelP), NF-κB (e), and caspase (f) in the kidneys of adolescent rats. Representative western blots of these proteins (normalized to β-actin) are shown (g). The results are expressed as the mean ± SEM and were analyzed by multifactorial analysis of variance (one-way ANOVA) followed by Tukey’s test. The number of animals in each group was 8. Groups: C, control group; BD, binge-drinking group; CSe, control selenium-supplemented group; BDSe, binge-drinking selenium-supplemented group. Significant differences between groups are expressed as p values and are indicated by the asterisk for comparisons between the C and BD groups, by the superscript ‘a' for comparisons between the BD and BDSe groups, by the superscript ‘c' for comparisons between the C and CSe groups, and by the superscripted solid circle for comparisons between the CSe and BDSe groups
SBP was increased in BD rats; BD rats also presented reduced serum GPx activity and elevated serum lipid oxidation values (Fig. 3). Se supplementation to these animals partially decreased SBP, increased serum GPx activity and partially reduced serum lipid oxidation. Se supplementation to control animals increased serum GPx activity but did not increase SBP or lipid oxidation.
Systolic blood pressure (a) and oxidative balance in serum [GPx activity (b) and lipid (c) and protein (d) oxidation]. The results are expressed as the mean ± SEM and were analyzed by multifactorial analysis of variance (one-way ANOVA) followed by Tukey’s test. The number of animals in each group was 8. Groups: C, control group; BD: binge-drinking group; CSe, control selenium-supplemented group; BDSe, binge-drinking selenium-supplemented group. Significant differences between groups are expressed as p values and are indicated by the asterisk for comparisons between the C and BD groups, by the superscript ‘a’ for comparisons between the BD and BDSe groups, by the superscript c for comparisons between the C and CSe groups, and by the superscripted solid circle for comparisons between the CSe and BDSe groups
Creatinine, Na+, K+, urea, and Se levels in serum and urine were measured together with their relative renal clearance values (Table 2). BD increased serum Na+ and decreased serum K+ and Se concentrations. Se supplementation slightly decreased Na+ serum values and greatly increased Se concentrations. BD decreased Se concentrations in urine, while Se supplementation decreased urea concentration and increased Se elimination via urine. With respect to relative clearances, BD significantly decreased Na+ and Se clearance and increased K+ clearance. Se supplementation did not modify Na+ or K+ relative clearance, but it did increase the CLSe/CL creatinine ratio. Se supplementation to control animals only modified Se parameters; all of these parameters were increased in Se-supplemented control animals compared to nonsupplemented control animals.
Renal functional parameters were greatly affected by exposure to BD (Fig. 4). BD decreased creatinine clearance and FENa and increased TTKG and serum Aldo levels; it also drastically reduced relative Aldo clearance. The albumin/creatinine ratio remained, however, unaltered. Se supplementation to BD animals slightly increased EFNa and decreased TTKG and serum Aldo levels. While it did not modify creatinine clearance, Se supplementation also greatly increased relative Aldo clearance. Se supplementation to control animals only partially decreased TTKG values from their control levels.
Renal function parameters: creatinine clearance (a), albumin/creatinine ratio (b), fractional excretion of Na+ (FENa) (c), transtubular K+ excretion (TTKG) (d), serum aldosterone levels (e), and aldosterone clearance (f). The results are expressed as the mean ± SEM and were analyzed by multifactorial analysis of variance (one-way ANOVA) followed by Tukey’s test. The number of animals in each group was 8. Groups: C, control group; BD, binge-drinking group; CSe, control selenium-supplemented group; BDSe, binge-drinking selenium-supplemented group. Significant differences between groups are expressed as p values and are indicated by the asterisk for comparisons between the C and BD groups, by the superscript ‘a’ for comparisons between the BD and BDSe groups, by the superscript ‘c’ for comparisons between the C and CSe groups, and by the superscripted solid circle for comparisons between the CSe and BDSe groups
Discussion
Despite the fact that BD rats consumed the same amount of Se as control rats and that they exhibited reabsorption of Se from urine, which decreased Se clearance, serum Se values and kidney deposits were significantly decreased in BD rats compared to control rats, confirming the fact that repeated acute ethanol exposure drastically reduces Se bioavailability. This effect could be due to the use of this element by the body to synthetize active GPx selenoprotein to fight against acute ethanol-induced oxidative stress. This explanation is consistent with the lower GPx activity and higher oxidation in the serum and kidneys and with the lower GPx-1, GPx-3, and GPx-4 expression in the kidneys of BD animals than in control animals (Fig. 5). Moreover, given that the activity of the rest of the antioxidant enzymes was increased in the kidneys, Se and GPx seem to play important, specifically antioxidant, roles after BD exposure. This exclusive GPx alteration is not observed in other oxidative stress-related renal conditions, such as renal insufficiency, in which CAT [27] and SOD activity is decreased [28] while GPx activity remained unaltered. It is known that intermittent BD during adolescence specifically reduces the hepatic levels of glutathione (GSH), which is necessary for GPx to function correctly [15]. Moreover, previous studies measuring hepatic antioxidant enzyme activity have observed only GPx activity to be decreased [25]. In this study, kidney GR activity in BD rats was markedly increased, implying increased GSH generation. GPx decreased significantly, however, pointing to kidney Se depletion as a key factor in the dysregulation of GPx activity and oxidative balance.
When extra Se was supplied to BD rats, serum Se levels and kidney deposits increased. Therefore, GPx activity in these locations completely prevented lipid and protein oxidation in the kidneys and partially prevented lipid oxidation in serum. This finding demonstrates that Se homeostasis is of great importance for combatting the oxidative stress generated by BD exposure, especially in the kidneys.
Se supplementation also increased the kidney expression of GPx-1, GPx-3, and GPx-4, with GPx-3 having the greatest upregulation (Figs. 2 and 5). It is worth noting that this GPx is mainly synthetized in kidney tissue and that it acts against ROS in the kidneys [18] and in plasma [17]. The increased expression of this selenoprotein is essential to prevent biomolecule oxidation in the above tissues. As expected, Se supplementation increased the activity of the GPxs, which had decreased in the kidneys and serum after exposure to BD. The increased GPx activity found in the kidneys of Se-supplemented BD rats could also be due in part to the increase in GPx1 expression.
With regard to GPx4 expression, it is important to remember that this is the only selenoprotein associated with the protection of biomembranes and therefore, mitochondria [29], from oxidative insults. It has been shown in studies with transgenic mice that GPx4 inhibits oxidative-stress-induced cyt. c release from mitochondria and the induction of apoptosis [30]. Moreover, there seems to be a relationship between GPx4 and NF-κB in different cell cultures [31,32,33]. NF-κB plays a crucial role in the decision between life and death in cells. NF-κB activation exerts an antiapoptotic effect by which it protects cells from apoptosis during tissue injury. When NF-κB activation decreases, however, apoptosis is activated [31]. In the liver of adolescent rats, it has been observed that intermittent BD exposure downregulates GPx4 and NF-κB expression and upregulates caspase-3 expression and apoptosis [16].
Similar to the case in the liver, in this study, both GPx4 and NF-κB expression was decreased and caspase-3 expression was increased in adolescent BD rat kidneys (Figs. 2 and 5). Only a few studies have analyzed inflammatory and apoptotic effects in the kidneys after ethanol exposure. Lu et al. [34] found that acute ethanol exposure in in vitro podocyte cultures only generated oxidative stress in these renal cells if the increase in H2O2 was very high because podocytes have a very efficient antioxidant system against this particular ROS. The authors found that in these cells, ethanol-induced apoptosis via Ca2+ influx through the transient receptor potential canonical channel 6, which affected mitochondria and the intrinsic apoptotic pathway via caspase-9 [35]. Ethanol, however, induces oxidative kidney injury mainly in tubular epithelial cells [36]. Latchoumycandane et al. [37] also found that chronic ethanol intake in rats mainly increases apoptosis in tubular epithelial cells, especially in the distal tubular wall, through a mechanism related to oxidative stress and caspase-3 activity. This effect probably occurs by the intrinsic apoptosis pathway, which is related to the release of cyt.c into the cytosol by mitochondrial oxidation. Latchoumycandane et al. [36] also found a decrease in GFR after ethanol exposure and explained that this reduction mainly compromised renal filtration rate through neutrophilic inflammation caused by ethanol, since neutrophil infiltration and activation lead to myeloperoxidase-dependent oxidation and damage to kidney function more than to direct oxidative effects on podocytes [37]. Until now, however, little has been known about BD exposure, kidney inflammation, or NF-κB expression.
Se supplementation to BD rats increased the kidney expression of NF-κB p65 to values similar to control values, probably leading to an apoptotic balance. These results coincide with elevated GPx4 expression that could reduce oxidative stress in kidney mitochondria, favoring mitochondrial survival and decreasing the levels of cyt. c released from mitochondria into the cytosol, therefore preventing apoptosis. Similar results were found in liver tissues of BD-exposed Se-supplemented rats [16].
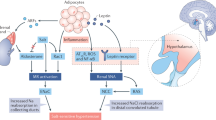
The observed changes in oxidative balance and GPx, NF-κB p65, and cleaved caspase-3 expression after BD could be related to kidney functional alterations. BD reduced GFR, as demonstrated by reduced creatinine clearance and urinary flow; this reduction in GFR indicates a water reabsorption process. Moreover, BD exposure caused hypernatremia, hypokalemia and hyperaldosteronemia together with high renal Na+ reabsorption, high urinary K+ secretion, and even a reduction in urinary Aldo excretion associated with a reduction in relative Aldo clearance (Figs. 4 and 5). It has been demonstrated that the neural circuits involved in the control of both ethanol and Na+ consumption behaviors share common pathways and neurochemical systems [38] and that high systemic ROS production caused by BD exposure stimulates the SNS, HHA, and RAAS, contributing to increases in the levels of the antinatriuretic hormone Aldo [2]. For all of these reasons, in this study, BD consumption during adolescence (at which time the RAAS is especially active [39]) led to an important osmotic and ionic disturbance that, together with systemic oxidative stress, caused an increase in SBP. Therefore, it is clear that the kidneys play an important role in the genesis of HTN in BD animals.
By increasing GPx expression and activity mainly in the kidneys, the antioxidant Se improved inflammatory status and alleviated apoptosis in BD animal kidneys, improving some of their functions—mainly those related to electrolyte balance, since Se partially decreased Na+ and serum Aldo levels by partially increasing renal function, thus improving FENa and TTKG, and increasing Aldo clearance. It is known that lipid oxidation leads to changes in membrane composition, interfering with carrier functions such as Na+/K+-ATPase activity and increasing Na+ reabsorption and K+ excretion [8], especially in renal papillary collecting duct cells [40]. Moreover, Rodrigo and Rivera [10] suggest that ethanol treatment enhances the upregulation of Na+K+-ATPase activity both by lipid oxidation and by Aldo. In this study, Se supplementation reduced lipid renal oxidation and serum Aldo levels, improving electrolyte balance. Se reduced serum Aldo levels by increasing its renal excretion. Although this mechanism is not understood, supplementation of chronic ethanol-exposed rats with other antioxidants, such as folic acid, has previously been observed to produce the same effect [41]. Since Se partially reduces systemic oxidative stress produced by BD exposure, Se could decrease the stimulation of the SNS and RAAS and therefore decrease serum Aldo levels, contributing to improving electrolyte balance and SBP. Moreover, the lack of renal Aldo clearance could have a hepatic origin, since Aldo must be metabolized in the liver in order to be soluble for excretion in urine. Aldo hepatic metabolism first requires cytochrome P450 and then GSH. Both of these compounds are consumed in great quantities through hepatic oxidative metabolism [42]. Se improves hepatic oxidative balance and AST levels after BD exposure [16], contributing to improving hepatic Aldo metabolism and therefore improving renal Aldo clearance. However, functions related to water reabsorption and filtration processes were not improved by Se supplementation. This could be because the oxidative stress generated by BD exposure seems mainly to affect distal tubular epithelial cells and not glomerular podocytes and because the reduction in GFR is mainly related to inflammatory processes [37].
In summary, BD exposure triggers kidney osmotic and ionic imbalances, which contribute to increasing SBP. These disturbances could be related in part to Se and selenoprotein GPxs, which contribute to reducing systemic oxidative stress and oxidative, inflammatory and apoptotic alterations in the kidneys. Se supplementation is indicated as a good strategy for preventing total lipid and protein oxidation in the kidneys via GPx activity, for increasing hepatic expression of NF-κB (a factor intimately related to apoptosis and immune function) and for decreasing caspase-3 activation, which improves ionic disturbances. In turn, these effects improve serum Aldo levels and SBP. However, Se supplementation does not improve the low GFR or low urinary flow caused by BD exposure, probably because glomeruli are affected less by oxidative stress than by neutrophilic infiltration.
References
Martinotti G, Lupi M, Carlucci L, Santacroce R, Cinosi E, Acciavatti T, et al. Alcohol drinking patterns in young people: a survey-based study. J Health Psychol. 2017;22:1889–96. https://doi.org/10.1177/1359105316667795.
Piano MR, Mazzuco A, Kang M, Phillips SA. Cardiovascular consequences of binge drinking: an integrative review with implications for advocacy, policy, and research. Alcohol Clin Exp Res. 2017;41:487–96. https://doi.org/10.1111/acer.13329.
Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–33. https://doi.org/10.1161/01.HYP.0000052314.95497.78.
González J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: new insights. World J Cardiol. 2014;6:353–66. https://doi.org/10.4330/wjc.v6.i6.353.
Kumar SD, Vasudevan DM. Alcohol induced effects on kidney. Indian J Clin Biochem. 2008;23:4–9. Accessed 1 Jun 2018
Matsumoto A, Nagasawa Y, Yamamoto R, Shinzawa M, Hasuike Y, Kuragano T, et al. The association of alcohol and smoking with CKD in a Japanese nationwide cross-sectional survey. Hypertens Res. 2017;40:771–8. https://doi.org/10.1038/hr.2017.25.
Dinu D, Nechifor MT, Movileanu L. Ethanol-induced alterations of the antioxidant defense system in rat kidney. J Biochem Mol Toxicol. 2006;19:386–95. https://doi.org/10.1002/jbt.20101.
Rodrigo R, Thielemann L, Olea M, Muñoz P, Cereceda M, Orellana M. Effect of ethanol ingestion on renal regulation of water and electrolytes. Arch Med Res. 1998;29:209–18. http://www.ncbi.nlm.nih.gov/pubmed/9775453 Accessed 1 Jun 2018
Amet Y, Plée-Gautier E, Berthou F, Adas F, French SW. Adaptation to chronic ethanol administration emphasized by fatty acid hydroxylations in rat liver and kidney microsomes. Eur J Nutr. 2000;39:270–6. http://www.ncbi.nlm.nih.gov/pubmed/11395987 Accessed 1 Jun 2018
Rodrigo R, Rivera G. Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radic Biol Med. 2002;33:409–22. http://www.ncbi.nlm.nih.gov/pubmed/12126763 Accessed 1 Jun 2018
Assadi FK. Acute effect of ethanol on renal electrolyte excretion in rats. Alcohol. 2018;6:257–60. http://www.ncbi.nlm.nih.gov/pubmed/2660849 Accessed 1 Jun 2018
Kim H-N, Kim S-H, Song S-W. Is alcohol drinking associated with renal impairment in the general population of South Korea? Kidney Blood Press Res. 2014;39:40–49. https://doi.org/10.1159/000355775.
Yang X, Li Y, Li Y, Ren X, Zhang X, Hu D, et al. Oxidative stress-mediated atherosclerosis: mechanisms and therapies. Front Physiol. 2017;8. https://doi.org/10.3389/fphys.2017.00600.
Carreras O, Ojeda ML, Nogales F. Chapter 11 – Selenium dietary supplementation and oxidative balance in alcoholism. In: Molecular aspects of alcohol and nutrition. 2016. p. 133–42. https://doi.org/10.1016/B978-0-12-800773-0.00011-2.
Ojeda ML, Rua RM, Murillo ML, Carreras O, Nogales F. Binge drinking during adolescence disrupts se homeostasis and its main hepatic selenoprotein expression. Alcohol Clin Exp Res. 2015;39:818–26 https://doi.org/10.1111/acer.12707.
Ojeda ML, Carreras O, Sobrino P, Murillo ML, Nogales F. Biological implications of selenium in adolescent rats exposed to binge drinking: oxidative, immunologic and apoptotic balance. Toxicol Appl Pharmacol. 2017;329:165–72. https://doi.org/10.1016/j.taap.2017.05.037.
Burk RF, Olson GE, Winfrey VP, Hill KE, Yin D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am J Physiol Liver Physiol. 2011;301:G32–G38. https://doi.org/10.1152/ajpgi.00064.2011.
Olson GE, Whitin JC, Hill KE, Winfrey VP, Motley AK, Austin LM, et al. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am J Physiol Ren Physiol. 2010;298:F1244–53. https://doi.org/10.1152/ajprenal.00662.2009.
Hoffmann PR, Hoge SC, Li P-A, Hoffmann FW, Hashimoto AC, Berry MJ. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007;35:3963–73. https://doi.org/10.1093/nar/gkm355.
Brigelius-Flohé R, Flohé L. Selenium and redox signaling. Arch Biochem Biophys. 2017;617:48–59. https://doi.org/10.1016/j.abb.2016.08.003.
Ojeda ML, Nogales F, Murillo ML, Carreras O. Selenium or selenium plus folic acid-supplemented diets ameliorate renal oxidation in ethanol-exposed pups. Alcohol Clin Exp Res. 2012;36:404–12. https://doi.org/10.1111/j.1530-0277.2012.01788.x.
Ozkol H, Bulut G, Balahoroglu R, Tuluce Y, Ozkol HU. Protective effects of selenium, N-acetylcysteine and vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175:177–85. https://doi.org/10.1007/s12011-016-0762-8.
Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–23. http://www.ncbi.nlm.nih.gov/pubmed/11199278 Accessed 19 Jun 2018
National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004;3:3.
Nogales F, Rua RM, Ojeda ML, Murillo ML, Carreras O. Oral or intraperitoneal binge drinking and oxidative balance in adolescent rats. Chem Res Toxicol. 2014;27:1926–33. https://doi.org/10.1021/tx5002628.
Ojeda ML, Nogales F, Vázquez B, Delgado MJ, Murillo ML, Carreras O. Pharmacology and cell metabolism: alcohol, gestation and breastfeeding: selenium as an antioxidant therapy. Alcohol Alcohol. 2009;44:272–7. https://doi.org/10.1093/alcalc/agp004.
Sindhu RK, Ehdaie A, Farmand F, Dhaliwal KK, Nguyen T, Zhan CD, et al. Expression of catalase and glutathione peroxidase in renal insufficiency. Biochim Biophys Acta. 2005;1743:86–92. https://doi.org/10.1016/j.bbamcr.2004.08.013.
Vaziri ND, Lin C-Y, Farmand F, Sindhu RK. Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int. 2003;63:186–94. https://doi.org/10.1046/j.1523-1755.2003.00711.x.
Knopp EA, Arndt TL, Eng KL, Caldwell M, LeBoeuf RC, Deeb SS, et al. Murine phospholipid hydroperoxide glutathione peroxidase: cDNA sequence, tissue expression, and mapping. Mamm Genome. 1999;10:601–5. http://www.ncbi.nlm.nih.gov/pubmed/10341094 Accessed 1 Jun 2018
Liang H, Yoo S-E, Na R, Walter CA, Richardson A, Ran Q. Short form glutathione peroxidase 4 is the essential isoform required for survival and somatic mitochondrial functions. J Biol Chem. 2009;284:30836–44. https://doi.org/10.1074/jbc.M109.032839.
Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-kappaB: effects on liver homeostasis and beyond. Endocr Rev. 2007;28:365–86. https://doi.org/10.1210/er.2006-0031.
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. https://doi.org/10.1016/j.cell.2004.07.013.
Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J. 2000;351(Pt 1):183–93. http://www.ncbi.nlm.nih.gov/pubmed/10998361. Accessed 1 Jun 2018
Lu X-Y, Liu B-C, Wang L-H, Yang LL, Bao Q, Zhai YJ, et al. Acute ethanol induces apoptosis by stimulating TRPC6 via elevation of superoxide in oxygenated podocytes. Biochim Biophys Acta. 2015;1853:965–74. https://doi.org/10.1016/j.bbamcr.2015.01.007.
Huang H, You Y, Lin X, Tang C, Gu X, Huang M, et al. Inhibition of TRPC6 signal pathway alleviates podocyte injury induced by TGF-β1. Cell Physiol Biochem. 2017;41:163–72. https://doi.org/10.1159/000455985.
Latchoumycandane C, Nagy LE, McIntyre TM. Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation. Free Radic Biol Med. 2014;69:403–16. https://doi.org/10.1016/j.freeradbiomed.2014.01.001.
Latchoumycandane C, Nagy LE, McIntyre TM. Myeloperoxidase formation of PAF receptor ligands induces PAF receptor-dependent kidney injury during ethanol consumption. Free Radic Biol Med. 2015;86:179–90. https://doi.org/10.1016/j.freeradbiomed.2015.05.020.
Godino A, Abate P, Amigone JL, Vivas L, Molina JC. Prenatal binge-like alcohol exposure alters brain and systemic responses to reach sodium and water balance. Neuroscience. 2015;311:92–104. https://doi.org/10.1016/j.neuroscience.2015.10.004.
Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. https://doi.org/10.1016/j.alcohol.2011.08.005.
Rodrigo R, Trujillo S, Bosco C, Orellana M, Thielemann L, Araya J. Changes in (Na+K)-adenosine triphosphatase activity and ultrastructure of lung and kidney associated with oxidative stress induced by acute ethanol intoxication. Chest. 2002;121:589–96. http://www.ncbi.nlm.nih.gov/pubmed/11834676 Accessed 1 Jun 2018
Barrero MJ, Ojeda ML, Díaz Castro J, Nogales F, Murillo ML, Carreras O. The effects of ethanol upon hydric balance and arterial pressure in rats: folic acid as a possible hypotensor. Life Sci. 2012;90:337–42. https://doi.org/10.1016/j.lfs.2011.12.008.
Ojeda L, Nogales F, Murillo L, Carreras O. The role of folic acid and selenium against oxidative damage from ethanol in early life programming: a review. Biochem Cell Biol. 2018;96:178–188. https://doi.org/10.1139/bcb-2017-0069.
Acknowledgements
Grants from the Andalusian Regional Government supporting the CTS-193 research group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sobrino, P., Ojeda, M., Nogales, F. et al. Binge drinking affects kidney function, osmotic balance, aldosterone levels, and arterial pressure in adolescent rats: the potential hypotensive effect of selenium mediated by improvements in oxidative balance. Hypertens Res 42, 1495–1506 (2019). https://doi.org/10.1038/s41440-019-0265-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0265-z
Keywords
This article is cited by
-
Binge drinking leads to an oxidative and metabolic imbalance in skeletal muscle during adolescence in rats: endocrine repercussion
Journal of Physiology and Biochemistry (2023)