Abstract
Hypertension has a close affinity to brain degeneration and cognitive decline during the aging process. The default mode network (DMN) is usually affected in various diseases related to cognitive impairment (CI). The present research aimed to explore the alterations in the DMN and its subcomponents in hypertensive patients with and without CI and to investigate the associations between cognitive performance and network abnormalities. Resting-state functional magnetic resonance imaging and neuropsychological tests were performed in 74 subjects, namely, 30 hypertensive patients with normal cognition (HTN-NC), 25 hypertensive patients with CI (HTN-CI), and 19 healthy controls. Seed-based functional connectivity (FC) analysis was performed to identify the DMN patterns. The group differences in the DMN were mainly shown in brain regions related to the core subsystem and the dorsal medial subsystem of the DMN. Post hoc analysis revealed a trend of dissociation among the DMN subsystems in the HTN-NC group. In contrast, the HTN-CI group displayed extensively increased FC in both subsystems. Importantly, increased FC of the dorsal medial subsystem in the HTN-CI patients was associated with poor cognitive performance, such as scores on Mini-Mental State Examination (ρ = −0.438, P = 0.029) and Montreal Cognitive Assessment (ρ = −0.449, P = 0.025). The findings suggest that extensively increased connectivities in the core subsystem and the dorsal media subsystem of the DMN may distinguish hypertension with CI from hypertension with normal cognition. The characteristic change in the dorsal medial subsystem may become an early imaging biomarker for the diagnosis and treatment of cognitive impairment associated with hypertension.
Similar content being viewed by others
Introduction
Hypertension remains a worldwide public health problem due to its high prevalence. In China, hypertension accounted for nearly 1/3 of the total deaths and ~1/6 of the total disability-adjusted life years in 2013, mainly because of its contributions to cardio-cerebral vascular diseases [1]. Furthermore, hypertension can act as an independent risk factor in the development of cognitive decline, Alzheimer’s disease (AD), and vascular dementia [2,3,4].
White matter (WM) lesions are commonly observed in hypertensive patients [5], and these lesions are supposed to disrupt the inner links of brain structures, leading to cognitive impairment. Brain atrophy [6] and cerebral amyloid β-protein deposition [7] are also associated with hypertension. The imbalance of cerebral blood flow perfusion in the frontal and parietal lobes [8,9,10] may be another cause of hypertension-related cognitive impairment. However, the mechanisms underlying the development of cognitive impairment in hypertension remain relatively unclear, and predicting cognitive impairment in hypertensive patients is still difficult.
A series of functional resting-state networks (RSNs) have been identified based on the temporal correlations between fluctuations in blood oxygen level-dependent signals across brain regions, also known as functional connectivity (FC) [11]. The default mode network (DMN) is one of the most important and widely studied networks. The DMN plays an active role in complicated cognitive processing, and abnormal alterations of the DMN have been shown in various neuropsychological diseases related to cognitive impairment, such as AD [12] or other types of dementia [13], depression [14], and schizophrenia [15]. The DMN is commonly subdivided into three subcomponents or subsystems, namely, the core subsystem, the medial temporal subsystem, and the dorsal medial subsystem [16]. Each subsystem has several central hubs that are involved in complex cognitive functions [17]. The three subsystems interact as a unit to maintain normal cognition and self-generated experience [18], and the subsystems are usually recruited during unconstrained resting-state periods.
Both disruption in the equilibrium and disassociation of the DMN subcomponents are observed in those with AD, type 2 diabetes mellitus (T2DM), major depression, and the apolipoprotein E (ApoE) type 4 allele [19,20,21,22,23]. However, the changing patterns of the DMN subcomponents and the associations of these changes with cognitive impairment in hypertensive patients are still unclear. There have even been a limited number of studies on the alterations in RSNs in hypertensive patients; only Li et al. [24] found that defective WM and impaired functional connectivity of frontoparietal networks may account for the underlying mechanisms of cognitive impairment in hypertensive patients. Therefore, it is necessary to explore the impaired characteristics of the DMN in hypertensive patients.
In the present study, hypertensive patients with normal cognition (HTN-NC), hypertensive patients with cognitive impairment (HTN-CI) and healthy controls underwent multimodal MRI scans and neuropsychological tests. DMN patterns were identified in each group. The present study aimed to explore the FC alterations in the DMN of hypertensive patients with and those without cognitive impairment and to determine probable imaging markers for the prediction of cognitive impairment in hypertensive patients. We hypothesized that a trend of disassociation of the DMN subsystems would be observed in hypertensive patients and that altered DMN patterns would be disparate between hypertensive patients with and without cognitive impairment.
Methods and materials
Participants
This study was approved by the Nanjing Drum Tower Hospital Ethics Committee in January 2017. The subjects of this research were recruited from among the outpatients and inpatients in our department of neurology. All the participants gave written informed consent prior to participation. All subjects underwent neuropsychological tests, MRI scanning, and computed tomography angiography (CTA) or B-mode ultrasonography of the intracranial and carotid arteries.
All the patients in the hypertensive group were over 50 years old, were diagnosed by the same experienced physician and had a history of taking oral antihypertensive drugs based on their medical records. Based on the standards described in the guidelines [25], each hypertensive patient was classified into different hypertension levels at the same time. The control group included cognitively normal people (age >50 years) without any history of high blood pressure. The exclusion criteria of this research were as follows: (1) dementia (e.g., AD, stroke-related dementia, and other types of dementia), depression and other mental disorders; (2) a long history of taking hypnotic sedative drugs and psychotropic drugs; (3) acute or subacute ischemic stroke and cerebral hemorrhage or a history of stroke (with a diameter of infarction larger than 15 mm on MRI); (4) a history of alcohol dependence, Parkinson’s disease, epilepsy, and thyroid gland disease; (5) more than 5 cerebral microbleeds (CMBs) or severe WM lesions (reaching the degree of Fazekas III according to the Fazekas scale [26]); (6) contraindications of MRI scanning or poor quality of the images; and (7) severe intracranial artery or carotid artery stenosis (larger than 50% stenosis or occlusion of the responsible artery). To guarantee the congruity of the images, all the images in this study were collected via the same MRI machine.
Neuropsychological evaluation
A professional neuropsychologist performed the neuropsychological tests before or on the day of the MRI scanning. Physical examinations were also conducted to determine positive neurological signs. The Chinese translation of the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) served as the general cognitive tests. Subjects with cognitive impairment were distinguished from subjects with normal cognition by the MMSE and MoCA scores and their educational experience. The cut-offs of cognitive impairment for the MMSE were made based on the results of the Beijing study (Table 1) [27]. Due to the low specificity of identifying cognitively normal people by using 25/26 as a cut-off for individuals with 12 years or less of education, the normative data for the MoCA were formulated according to several studies in the population in China (Table 1) [28, 29]. The subjects with scores lower than or equal to the cut-off points of MMSE or MoCA were defined as cognitive impairment (Table 1). According to the status of general cognitive function, the hypertensive patients were divided into an HTN-NC group (n = 30) and an HTN-CI group (n = 25). Furthermore, 19 healthy subjects with normal cognition served as a control group.
Memory was assessed using the visual reproduction (VR)–long-delayed recall portion of the Wechsler Memory Scale (WMS) and the Auditory Verbal Learning Test (AVLT) delayed recall test. Language was assessed using the category verbal fluency (VBF) test and the Boston Naming Test (BNT). Processing speed was assessed using the Trail Making Test A (TMT-A) and the Stroop-B test. Executive function was evaluated using the Trail Making Test B and the Stroop-C test. Visuospatial ability and attention were evaluated using the visual reproduction–copy (VR-copy) portion of the Wechsler Memory Scale and the Clock Drawing Test (CDT).
MRI data acquisition
All subjects were scanned using a Philips Ingenia 3.0-T scanner (Philips, Eindhoven, Netherlands) with a 32-channel head coil. See Supplementary Information for details.
Image preprocessing
The imaging data were analyzed using the toolbox for Data Processing & Analysis for Brain Imaging (DPABI) V2.3 (http://rfmri.org/dpabi). See Supplementary Information for details.
White matter hyperintensity segmentation and quantification
For the analysis of the WM, gray matter (GM), and CSF volumes, three-dimension fast field echo (3D FFE) images were segmented using SPM8 (statistical parametric mapping, http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox. The Wisconsin White Matter Hyperintensities Segmentation Toolbox (W2MHS) [30] was used to extract and analyze the total white matter hyperintensity (WMH), periventricular WMH, and deep WMH volumes. Taking into account individual cerebral differences, the adjusted WMH was computed with the proportion of the total WMH accounting for the sum of the WM and GM.
Definition of the DMN
A 5-mm-radius sphere in the posterior cingulate cortex (PCC) hub [Montreal Neurological Institute (MNI) space: −2, −45, 34] served as a seed region. The mean time series of the seed region was calculated as the reference time course for each participant. The correlation coefficient of each voxel was computed by a Pearson cross-correlational analysis between the seed time course and the time course of each voxel in the whole brain. To obtain the eventual individual maps of the DMN, Fisher’s z-transformation was applied in the calculation of the approximately normally distributed correlation coefficients by the equation: \(z = 0{\mathrm{.5}} \times {\mathrm{ln}}\frac{{{\mathrm{1 + r}}}}{{{\mathrm{1 - r}}}}\). Last, the individual maps of each network were acquired. The one sample t-test was used to identify the brain regions that positively correlated with each seed. The thresholds were set at a corrected P < 0.001, determined by Monte Carlo simulations for multiple comparisons (voxelwise P < 0.001, FWHM = 6 mm, cluster size > 486 mm3).
Statistical analysis
Demographic, clinical, and neuropsychological data
SPSS 23.0 (statistical program for social sciences, SPSS Inc. Chicago, IL) was used to perform all the statistical procedures. Comparisons of age, gender, and some demographic and neuropsychological data were performed by one-way analysis of variance (ANOVA) or the Χ2 test. Some categorical data, such as hypertension level and the existence of lacunar infarctions (LIs) and CMBs, were also compared by the chi-square test. The Kruskal–Wallis test was applied in the comparisons of nonnormally distributed demographic and neuropsychological data. Multiple linear regression was also used, controlling for gender, years of education, and age of all subjects. The results are presented in Supplementary Table 1. The threshold for statistical significance was set at P < 0.05.
FC analysis
All the functional MRI data were analyzed by REST (http://restingfmri.sourceforge.net) and DPABI V2.3. To analyze the DMN FC differences among the 3 groups, an ANCOVA was performed on the individual normalized maps in a voxelwise manner within a GM mask. Age, gender, years of education, head motion parameters, and the modulated GM maps obtained from the T1 segmentation were included as covariates in all the functional data analyses. The threshold was set at a corrected P < 0.05, as determined by Monte Carlo simulations (AlphaSim program in AFNI, http://afni.nih.gov/afni/docpdf/AlphaSim.pdf), for multiple comparisons in the whole brain (voxelwise P < 0.01, FWHM = 6 mm, cluster size >999 mm3).
The average FC strength of each significant region of interest (ROI) was extracted from each subgroup by REST 1.7 and was analyzed by SPSS 23.0 afterwards. Post hoc tests (significance was set at <0.05) were performed to explore the differences in FC among the subgroups. Spearman correlational analyses were performed in a ROIwise manner to explore the associations between the DMN FC values and cognitive performance and WMH volume in the hypertensive patients.
Results
Demographic and neuropsychological characteristics
No significant differences in gender, education, WMH volumes, hypertension duration, hypertension level, the number of LIs, the number of CMBs, or the number of subjects with T2DM (Table 2) were shown among the three groups. However, the individuals in the control group were significantly younger than those in hypertensive groups (P < 0.001, Table 2). The results of multiple linear regression analysis controlling for age, gender, and education (Supplementary Table 1) were in accordance with the results in Table 2. Significant differences in the MoCA, Stroop-B, and VR-delayed recall scores were shown among the three groups (Table 2, Supplementary Table 1). Specifically, the patients in the HTN-CI group had significantly lower scores on the MoCA than those in the other two groups (P < 0.001).
DMN activity in hypertensive patients
A typical spatial pattern of the DMN similar to that observed in a previous study with healthy normal subjects [31] was presented in all groups and included the anterior cingulate cortex, medial prefrontal cortex, lateral prefrontal cortex, precuneus, PCC, and medial temporal lobe (Fig. 1).
The DMN were constructed in the control group, HTN-NC group, HTN-CI group separately by the one sample t-test. The thresholds were set at a corrected P < 0.001, determined by Monte Carlo simulation for multiple comparisons. DMN default mode network, HTN-NC hypertensive patients with normal cognition, HTN-CI hypertensive patients with cognitive impairment
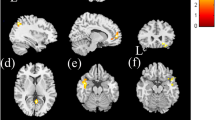
The comparison among the control, HTN-NC and HTN-CI groups showed that the ROIs with significant differences were mainly found in the frontal cortex, parietal cortex, and cingulate cortex (Table 3 and Fig. 2). Importantly, based on the division of the brain regions and their associated DMN subsystems in previous studies [16, 32], these regions mainly belonged to the core subsystem and the dorsal medial subsystem (Table 3). Since the hypertensive subjects were significantly older than control subjects, the effect of age on the ROIs with significant differences was investigated with and without age as a covariate, and the results were approximately similar (see Supplementary Fig. 1).
The DMN FC differences among the three groups. According to the ANCOVA, the 11 brain regions with significant differences were mainly located in the frontal cortex, parietal cortex, and cingulate cortex. The thresholds were set at a corrected P < 0.05, determined by Monte Carlo simulation for multiple comparisons (voxelwise P < 0.01, FWHM = 6 mm, cluster size > 999 mm3). L left, R right, DMN default mode network, FC functional connectivity, ANCOVA analysis of covariance
Post hoc analysis: As shown in Fig. 3a, b, compared with the control group, the HTN-NC group displayed a trend of dissociation among the DMN subsystems, i.e., decreased FC in the dorsal medial subsystem (including the left middle temporal gyrus, bilateral precentral gyrus, and medial frontal gyrus) (Fig. 3a) and increased FC in the core subsystem (including the right superior frontal gyrus, right supramarginal gyrus, bilateral angular gyrus, and dorsal anterior cingulate cortex) (Fig. 3b). In contrast, the HTN-CI group displayed significantly increased FC in all regions, with group differences compared to the control group. Notably, although most of the FC intensity differences between the HTN-NC group and the control group were not significant, a tendency of disassociation among the DMN subsystems was evident in the HTN-NC group.
DMN FC differences among the three groups in (a) the dorsal medial subsystem and (b) the core subsystem. A tendency of dissociation among the DMN subsystems was found in the HTN-NC group, while an overall significant FC enhancement appeared in all the brain regions in the HTN-CI group. The significant differences between each pair of groups in different brain regions according to the post hoc analysis are marked (*P < 0.05; **P < 0.01; ***P < 0.001). L left, R right, DMN default mode network, FC functional connectivity, HTN-NC hypertensive patients with normal cognition, HTN-CI hypertensive patients with cognitive impairment
Brain-behavior association analysis in hypertensive patients with cognitive impairment
No significant correlations between the WMH volumes and the DMN FC values were observed in the HTN-CI group (Supplementary Table 2). The FC strength of the dorsal medial subsystem was significantly correlated with the cognitive performance of the HTN-CI patients (Fig. 4). Specifically, the FC values in left middle temporal gyrus were negatively correlated with the MMSE scores in the HTN-CI group (ρ = −0.438, P = 0.029). Significant negative correlations were also observed between the MoCA scores and the FC values in the left medial frontal gyrus (ρ = −0.449, P = 0.025), the BNT scores and FC values in the right precentral gyrus (ρ = −0.419, P = 0.037), and the BNT scores and the FC values in the right medial frontal gyrus (ρ = −0.415, P = 0.039).
Spearman correlation analysis was performed to detect the relationship between DMN FC and cognitive performance in the HTN-CI group. Increased functional brain activity was associated with worse cognitive performance. a The FC of the L middle temporal gyrus (BA22) had a negative correlation with MMSE score. b The FC of the L medial frontal gyrus was negatively correlated with MoCA score. c, d The FCs of the R precentral gyrus and the L medial frontal gyrus showed inverse correlations with BNT score. L left, R right, BA Brodmann area, DMN default mode network, FC functional connectivity, HTN-CI hypertensive patients with cognitive impairment, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, BNT Boston Naming Test
Discussion
The present study found a tendency of disassociation among the DMN subsystems in HTN-NC patients and extensively increased connectivities among the DMN subsystems in HTN-CI patients. The increased connectivities in the dorsal medial subsystem were associated with poor cognitive performance in the HTN-CI patients. These findings could contribute to the understanding of the mechanisms underlying cognitive impairment in hypertension and could provide potential imaging biomarkers for cognitive impairment related to hypertension.
Although there were no significant differences between the FC of the HTN-NC group and of the control group, there still existed a disassociation trend among the DMN subsystems, i.e., decreased FC in the dorsal medial subsystem and increased FC in the core subsystem. The disruption of the equilibrium among the DMN subcomponents has also been observed in other diseases, such as AD [19], major depression [20], and particularly T2DM [22]. The DMN subsystems act as a whole and interact in a dynamic equilibrium that is critical for the maintenance of normal cognition [33]. The small-world properties are remarkably impaired in AD patients [34], indicating disruption of brain networks and the inability to integrate brain networks. The present study is the first to show a tendency of dissociation among the DMN subsystems in hypertensive patients, suggesting that the internal balance and integrity of the DMN subsystems is disrupted by hypertension. However, the tendency of dissociation within the DMN was found only in hypertensive patients with normal cognition. Thus, it is possible that merely the trend of dissociation among the DMN subsystems would not confer to cognitive impairment in hypertensive patients.
HTN-CI patients showed significant FC increases in both the dorsal medial subsystem and core subsystem of DMN, and previous studies have revealed a similar compensatory phenomenon in MCI, AD [35,36,37], Parkinson’s disease [38], and schizophrenia [39]. This compensation can be seen as a process of brain reconstruction and brain functional remodeling due to its plasticity after damage to original neural networks, especially in the early phase of CI [36, 40]. It should be noted that the abnormally increased FC appeared in the preliminary stage, presented a declining trend with progression of the disease and vanished at the terminal stage of the disease [41]. To compensate for the cerebral damage concomitant with the cognitive decline, the impaired region of brain could reallocate the cognitive and neurological resources from the whole brain [35]. Therefore, the HTN-CI patients may have the chance to inhibit the aggravation and prevent cognitive impairment since they are still in the initial stage of CI. Recent neuroimaging studies have confirmed that DMN changes could predict therapeutic effects in psychiatric disorders such as schizophrenia [42]. Similarly, the compensatory phenomenon of the DMN in HTN-CI patients may also hold great potential value for providing information about early prediction of cognitive impairment prognosis and treatment in hypertensive patients.
Interestingly, the changing patterns in the dorsal medial subsystem were prominently different between the HTN-NC patients and the HTN-CI patients, indicating that the function of the dorsal medial subsystem plays a predominant role in the cognitive impairment of these hypertensive patients. Our investigations further found significant negative relationships between the dorsal medial subsystem and corresponding neuropsychological tests of overall cognitive function and language ability, such as the MMSE, MoCA, and BNT, in HTN-CI patients. These findings were in line with those of previous studies that found that the dorsal medial subsystem mainly participates in semantic processing and comprehension of conceptual knowledge components [17]. Thus, we postulated that the increased FC in the dorsal medial subsystem and its inverse correlations between the neuropsychological results is more closely related to the cognitive compensation for the damage to brain function and loss of manipulation in HTN-CI patients.
WMH is common in hypertensive patients. The structural basis of brain function is the neuronal connections among various regions via WM tracts [43]. However, the present study found no association between the DMN FC value and WMH grade or WMH volume in hypertensive patients, a finding that did not coincide with the assumption that WMH is the main factor that contributes to the FC alterations related to hypertension. The following are possible reasons. The microstructural integrity of the WM in particular regions could play a key role in specific cognitive disturbances [44]; however, the present volumes of the WMH presented only as a macroscopic lesion of the white matter tracts. It is clear that the occurrence of cognitive decline is the result of the interaction between brain structure and function, and more detailed and precise studies of the microstructure in WM tracts could lead to more reliable conclusions.
Limitations and future work
Several limitations need to be addressed. First, the sample size was relatively small for making the conclusions persuasive. Since the current study was a cross-sectional study, it is uncertain that the obtained results were causes of hypertension-induced cognitive impairment or the results of hypertension-induced cognitive impairment. Thus, we intend to enlarge the sample size and follow up subjects for three or four years to investigate the association between alterations of cognitive performances and those of brain network activity. The continuous follow-up of blood pressure fluctuations, cognition and DMN alterations of all subjects is underway.
Additionally, the diversity of antihypertensive drugs, hypoglycemic drugs, and lipid-lowering drugs were not analyzed in the present study. An effective blood pressure control can retard the speed of hypertension progression, and different drugs generate different therapeutic effects. No studies have specifically explored the effects of hypoglycemic drugs or lipid-lowering drugs on brain networks. Even the effects of those drugs on cognition are controversial. Both beneficial effects (cognitive protection [45] and reducing the risk of AD [46]) and neurotoxic effects (aggravating the cognitive impairment [47, 48]) were found in previous studies. The LIs and T2DM may have affected the present findings, although these confounding factors were comparable among the three groups. As it is difficult to control the effects of different drugs and other diseases, exclusion of those patients with LIs, T2DM, and hyperlipidemia in a future study would be a wise choice to alleviate the effects of different confounding factors.
Last but not least, the diagnosis of hypertension was based on medical records and history of taking oral antihypertensive drugs. The assessment of previous BP control is difficult in patients with long-term hypertension. Thus, home BP monitoring (HBPM) plays an important role in hypertension management and evaluation of BP control [25]. HBPM is more closely related to hypertension-induced organ damage than is clinical BP measurement [25], and the variability of home BP has been associated with cognitive decline in hypertensive patients [49, 50]. Since the variability could also be an important influencing factor of brain network activity and cognition, we would like to calculate the variability of home BP through the records of HBPM and 24-h ambulatory BP measurement in future follow-up visits.
Conclusion
Different brain network alterations in the core subsystem and the dorsal media subsystem of DMN may differentiate hypertensive patients with cognitive impairment from those with normal cognition. HTN-CI patients displayed extensively increased connectivities in the DMN subsystems that were associated with poor cognitive performance. The characteristic changes in the dorsal medial subsystem may become an early imaging biomarker for cognitive impairment in hypertensive patients.
References
Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–323.
Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disord. 2009;2:241–60.
Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59:780–6.
Ronnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40-year follow-up of a population-based cohort. Dement Geriatr Cogn Disord. 2011;31:460–6.
Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56.
Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, et al. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Human Hypertens. 2012;26:295–305.
Carnevale D, Mascio G, Ajmone-Cat MA, D’Andrea I, Cifelli G, Madonna M, et al. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging. 2012;33:e219–229. 205
Jennings JR, Heim AF, Sheu LK, Muldoon MF, Ryan C, Gach HM, et al. Brain regional blood flow and working memory performance predict change in blood pressure over 2 years. Hypertension. 2017;70:1132–41.
Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–54.
Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, et al. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–65.
Rosazza C, Minati L. Resting-state brain networks: literature review and clinical applications. Neurol Sci. 2011;32:773–85.
Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–42.
Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, et al. Model-free group analysis shows altered BOLD FMRI networks in dementia. Human Brain Mapp. 2010;30:256–66.
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84.
Yeo B, Krienen F, Sepulcre J, Sabuncu M, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Andrews-Hanna J, Smallwood J, Spreng R. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52.
Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human Brain Mapp. 2010;30:625–37.
Jones DT, Machulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524–31.
Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–7.
Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM, et al. Non-stationarity in the “resting brain’s” modular architecture. PLoS ONE. 2012;7:e39731.
Cui Y, Jiao Y, Chen HJ, Ding J, Luo B, Peng CY, et al. Aberrant functional connectivity of default-mode network in type 2 diabetes patients. Eur Radiol. 2015;25:3238–46.
Machulda M, Jones D, Vemuri P, McDade E, Avula R, Przybelski S, et al. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol. 2011;68:1131–6.
Li X, Liang Y, Chen Y, Zhang J, Wei D, Chen K, et al. Disrupted frontoparietal network mediates white matter structure dysfunction associated with cognitive decline in hypertension patients. J Neurosci. 2015;35:10015–24.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219.
Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–22.
Zhang ZX, Hong X, Li H. The mini-mental state examination in the Chinese residents population aged 55 years and over in the urban and rural areas of Beijing. Chin J Neurol. 1999;03:149–53.
Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. 2011;24:184–90.
Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012;12:156.
Ithapu V, Singh V, Lindner C, Austin BP, Hinrichs C, Carlsson CM, et al. Extracting and summarizing white matter hyperintensities using supervised segmentation methods in Alzheimer’s disease risk and aging studies. Hum Brain Mapp. 2014;35:4219–35.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 2014;205:376–82.
Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–70.
Zhu H, Zhou P, Alcauter S, Chen Y, Cao H, Tian M, et al. Changes of intranetwork and internetwork functional connectivity in Alzheimer’s disease and mild cognitive impairment. J Neural Eng. 2016;13:046008.
Qi Z, Wu X, Wang Z, Zhang N, Dong H, Yao L, et al. Impairment and compensation coexist in amnestic MCI default mode network. Neuroimage. 2010;50:48–55.
Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 2010;256:598–606.
Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, et al. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer’s disease. Behav Brain Res. 2009;197:103–8.
Zhang D, Liu X, Chen J, Liu B, Wang J. Widespread increase of functional connectivity in Parkinson’s disease with tremor: a resting-state FMRI study. Front Aging Neurosci. 2015;7:6.
Wang H, Zeng LL, Chen Y, Yin H, Tan Q, Hu D. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci Rep. 2015;5:14655.
Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504.
Liu Y, Yu C, Zhang X, Liu J, Duan Y, Alexander-Bloch AF, et al. Impaired long distance functional connectivity and weighted network architecture in Alzheimer’s disease. Cereb Cortex. 2014;24:1422–35.
Tregellas JR, Tanabe J, Rojas DC, Shatti S, Olincy A, Johnson L, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry. 2011;69:7–11.
van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–41.
Tuladhar AM, van Norden AG, de Laat KF, Zwiers MP, van Dijk EJ, Norris DG, et al. White matter integrity in small vessel disease is related to cognition. Neuroimage Clin. 2015;7:518–24.
Cheng C, Lin CH, Tsai YW, Tsai CJ, Chou PH, Lan TH. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol Ser A Biol Sci Med Sci. 2014;69:1299–305.
Li G, Shofer JB, Rhew IC, Kukull WA, Peskind ER, McCormick W, et al. Age-varying association between statin use and incident Alzheimer’s disease. J Am Geriatr Soc. 2010;58:1311–7.
Chapman LE, Darling AL, Brown JE. Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2016;42:316–27.
Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29:800–11.
Liu Z, Zhao Y, Zhang H, Chai Q, Cui Y, Diao Y, et al. Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertens Res. 2016;39:245–53.
Matsumoto A, Satoh M, Kikuya M, Ohkubo T, Hirano M, Inoue R, et al. Day-to-day variability in home blood pressure is associated with cognitive decline: the Ohasama study. Hypertension. 2014;63:1333–8.
Acknowledgements
This work was supported by the National Key Research and Development Program of P. R. China (No. 2016YFC1300500-504), National Natural Science Foundation of P. R. China (No. 81630028, 81671665), Natural Science Foundation of Jiangsu Province (No. BE2016610, BK20160071), Jiangsu Provincial Key Medical Discipline (No. ZDXKA2016020), and Jiangsu Provincial Key Medical Talents (No. ZDRCA2016085). This funding body did not take part in study design; processing, analysis, or interpretation of data; composition of the article; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Gu, Y., Liu, R., Qin, R. et al. Characteristic changes in the default mode network in hypertensive patients with cognitive impairment. Hypertens Res 42, 530–540 (2019). https://doi.org/10.1038/s41440-018-0176-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0176-4
Keywords
This article is cited by
-
Alterations in regional homogeneity and functional connectivity associated with cognitive impairment in patients with hypertension: a resting-state functional magnetic resonance imaging study
Hypertension Research (2023)
-
Disrupted topological organization of functional brain networks is associated with cognitive impairment in hypertension patients: a resting-state fMRI study
Neuroradiology (2023)