Abstract
Chymase is a major angiotensin-converting enzyme (ACE)-independent angiotensin convertase, and its expression is upregulated in the maternal vascular endothelium in preeclampsia, a hypertensive disorder in human pregnancy. Increased chymase-dependent angiotensin II generation has been reported in several cardiovascular diseases, including atherosclerosis and aneurysmal lesions. However, it remains unclear how chymase is activated. Histone modification is an important regulatory mechanism that controls gene expression. In this study, using a chymase overexpression cell model, we investigated the mechanisms of chymase activation to test our hypothesis that histone acetylation could promote endothelial chymase expression. Human umbilical vein endothelial cells were transfected with the chymase gene. Trichostatin A was used to inhibit histone deacetylases (HDACs). The expression levels of chymase, ACE, and HDACs were determined by western blotting. Our results showed that ACE was strongly expressed in control cells, but was significantly downregulated in cells transfected to express chymase. Strikingly, we also found that HDAC inhibition resulted in a dose-dependent increase in chymase expression but a dose-dependent decrease in ACE expression in cells transfected with the chymase gene. HDAC inhibition was confirmed by the decreased expression of HDAC1 and HDAC6 in cells treated with trichostatin A. Increased chymase expression associated with reduced histone deacetylase expression was further confirmed by immunostaining of subcutaneous adipose sections from women with preeclampsia. We conclude that aberrant HDAC expression/activity could disturb the balance between ACE and chymase expression in endothelial cells. Our results support the clinical importance of chymase as a new pharmacological target for cardiovascular disorders.
Similar content being viewed by others
Introduction
Angiotensin II is a major bioactive product of the renin–angiotensin system (RAS) that is converted from angiotensin I to angiotensin II by angiotensin-converting enzyme (ACE). ACE is present in endothelial cells throughout the body. Angiotensin II is a potent vasoconstrictor, and increased angiotensin II formation has been demonstrated to play fundamental roles in the pathophysiology and pathogenesis of many cardiac and vascular diseases, such as hypertension and arteriosclerosis. Angiotensin II promotes oxidative stress and inflammatory responses, stimulates matrix metalloproteinase (MMP) activation, and induces vascular endothelial dysfunction [1]. Therefore, ACE inhibition blockade of angiotensin II formation and angiotensin II receptor-1 (AT-1) blockers limit angiotensin II actions represent the primary therapies for the management of hypertensive disorders.
Chymase is a chymotrypsin-like serine protease that was originally found in the secretory granules of mast cells, and it has recently been implicated in several cardiovascular diseases, including heart failure, cardiac hypertrophy, and diabetes mellitus [2,3,4,5]. Like ACE, chymase can to convert angiotensin I to angiotensin II. Studies have shown that chymase is actively responsible for ~70–80% of the angiotensin II generation in human heart tissue [6]; Chymase is also a major ACE-independent source of angiotensin II in human arteries [7]. Therefore, it has been speculated that chymase activation would have profound effects on the cardiovascular system because of its downstream vasoconstrictor actions via the generation of angiotensin II and endothelin [8]. Although the role of chymase in the systemic vasculature is unclear under physiological conditions, the chymase-dependent angiotensin II-generating capacity was found to be increased in several cardiovascular diseases, including atherosclerosis and aneurysmal lesions [9]. Increased chymase expression was also detected in vascular endothelium in women with preeclampsia, a hypertensive disorder in human pregnancy [10, 11]. Additionally, results from animal studies also support chymase participation in the development of vascular diseases, such as cigarette smoke-induced pulmonary hypertension (in hamsters) [12], abdominal aortic aneurysms (in dogs) [13], and salt-induced hypertension (in mice) [14].
Chymase is now considered a novel pharmacological target in several cardiovascular diseases [2]. However, the underlying mechanisms of chymase activation in diverse cardiovascular diseases remain largely unknown. Histone modification has been studied as a major regulatory event that is involved in many different stages of gene control. In the present study, using a chymase overexpression model, we investigated the relationship between chymase and ACE expression in endothelial cells to test our hypothesis that histone acetylation promotes chymase expression and activation in vascular endothelial cells. Increased chymase expression associated with reduced histone deacetylase (HDAC) expression was demonstrated by the immunostaining of subcutaneous adipose sections from women with preeclampsia.
Materials and methods
Endothelial cell isolation and culture
Human umbilical vein endothelial cells (HUVECs) isolated from uncomplicated term-delivered placentas were used in the present study. The collection of placentas for HUVEC isolation was approved by the Institutional Review Board (IRB) at Louisiana State University Health Sciences Center-Shreveport (LSUHSC-S), Louisiana. HUVECs were isolated by collagenase digestion as previously described [15]. Isolated HUVECs were seeded into fibronectin (10 ng/ml) (Sigma, St. Louis, MO)-coated cell culture plates or flasks and were incubated with endothelial growth medium (Lonza, Walkersville, MD).
Construction of chym/ZsGreen1 vector and chymase gene transfer
Vector pZsGreen1-N1 (pZs) obtained from Clontech (Palo Alto, CA) was used to construct the chymase vector, pChym. Briefly, the open reading frame of human chymase was amplified from human cDNA by polymerase chain reaction (PCR) using oligonucleotide to create restriction sites for Nhe І and Kpn І at the 5ʹ and 3′ ends of the chymase sequence using the following primers: sense primer: 5′ -AGCGCTAGCACCATGCTGCTTCTTCCTCTCC-3′ antisense primer: 5′-GGTGGTACCCAATTTGCCTGCAGGATCTG-3′. The restriction sites for Nhe I (5′-GCTAGC-3′) and Kpn I (5′-GGTACC-3′) are underlined. The primers were designed based on NCBI accession # M64269 and were synthesized by Integrated DNA Technologies (IDT, Coralville, IA). PCR was performed using platinum pfx DNA polymerase (Invitrogen, Carlsbad, CA). The PCR product and pZsGreen1-N1 were digested with Nhe І and Kpn І (New England Lab, Ipswich, MA). After ligation, competent Ecoli-Top10 (Invitrogen) was transformed with the plasmid, and selected positive clones were amplified. The chymase sequence was verified by Mclab sequencing (South San Francisco, CA).
Chymase gene transfer
Chymase gene transfer was accomplished in passage 2–3 HUVECs using the Pulser XcellTM electroporation system (Bio-Rad, Hercules, CA) and was confirmed by immunofluorescence staining. Cells transfected with vector pZs only served as the control. Briefly, an 20-μg aliquot of plasmid DNA was mixed with 5 × 106 cells in 600 μL of buffer per 4-mm electroporation cuvette. Fixed electroporation was carried out using a capacitance of 950μf and 250 v. The cells transfected with pZs vector only were used as the control. Twenty-four hours after pChym gene transfer, the cells were treated with HDAC inhibitor. The treatment was carried out for 24 h, and total cellular protein was then collected and stored at −80 °C until analyzed by western blotting.
Protein expression
Protein expression for chymase and ACE was examined by western blotting. Briefly, 10-µg aliquots of total cellular protein per sample were subjected to electrophoresis (Mini-cell protein-3 gel running system; Bio-Rad) and then were transferred to nitrocellulose membranes. The membranes were then probed with a primary antibody against chymase (cat# 444904; Calbiochem, San Diego, CA) or ACE antibody (sc-121187; Santa Cruz, San Diego, CA), followed by a species-targeted secondary antibody. The bands were then visualized by enhanced chemiluminescence (ECL) (Amersham Corp, Arlington Heights, IL). The HDAC1 and HDA6 expression levels, were both determined. Antibodies for HDAC1 (cat# 5356) and HDAC6 (cat# 7558) were purchased from Cell Signaling Technology (Danvers, MA). β-Actin expression was also determined for each sample and was used to normalize the protein expression for each sample. The density of protein bands was scanned and analyzed by NIH Image program 1.16.
Subcutaneous fat tissue collection
Maternal subcutaneous adipose tissue was collected during cesarean section delivery from normotensive and preeclamptic pregnant women, as we previously described [11]. The collection of subcutaneous adipose tissue was approved by the IRB at LSUHSC-S, LA. Normotensive pregnancy was defined as pregnancy with a blood pressure < 140/90 mmHg and the absence of obstetric and medical complications. Preeclampsia was defined as follows: sustained systolic blood pressure ≥ 140 mmHg or a sustained diastolic blood pressure ≥ 90 mmHg on two separate readings; proteinuria measurement of 1 + or more on dipstick or 24-h urine collection with ≥ 300 mg of protein in the specimen. Smokers and patients with signs of infection were excluded. To avoid clinical phenotypic differences in preeclamptic patients, patients with HELLP syndrome (hemolysis, elevated liver enzyme, and low platelet count), diabetes and/or renal diseases were excluded. In this study, subcutaneous tissues from 12 pregnant women, 6 normal and 6 preeclamptic, were examined. Tissue sections from the study subjects had been used in our previously published work [11]. Table 1 presents the demographic data for the study subjects.
Immunohistochemistry
Fresh subcutaneous adipose tissue was fixed immediately with 10% formalin and then was embedded in paraffin. A standard immunohistochemistry staining procedure was performed. Briefly, a series of deparaffinization procedures were performed with xylene and a graded ethanol series. Antigen retrieval was performed by boiling tissue slides with 0.01 M citric buffer. Hydrogen peroxide was used to quench the endogenous peroxidase activity. After blocking, the sections were incubated with primary monoclonal antibodies against human antigens overnight at 4 °C. The antibody against chymase (cat# MA5-11717) was purchased from ThermoFisher Scientific (Rockford, IL); ACE (sc-12187), AT-1 (sc-1173), HDAC1 (sc-81598), HDAC3 (sc11419), and HDAC6 (sc-11420) antibodies were from Santa Cruz Biotechnology (San Diego, CA). Corresponding biotin-conjugated secondary antibodies and the ABC staining system (Santa Cruz) were used according to the manufacturer’s instructions. Stained slides were counterstained with Gill’s formulation hematoxylin. Tissue sections stained with isotype IgG were used as controls. All slides stained with the same antibody were processed at the same time. The stained tissue slides were reviewed under an Olympus microscope (Olympus IX71, Olympus Corporation, Tokyo, Japan), and images were captured by a digital camera and were recorded into a microscope-linked PC computer.
Statistical analysis
The data are expressed as the means ± standard error (SE). Statistical analysis was performed by paired t test or analysis of variance (ANOVA) using Prism 5 computer software (GraphPad Software, Inc. La Jolla, CA). The Student–NewmanKeuls test was used as a post-hoc test. A probability level less than 0.05 was considered statistically significant.
Results
Chymase overexpression downregulates ACE expression in endothelial cells
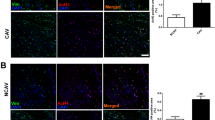
ACE was found to be present mainly in endothelial cells. To determine the consequences of chymase upregulation in endothelial cells, we constructed a vector that contains the human chymase gene (pChym) and transferred it into endothelial cells. The confirmation of chymase gene transfer in endothelial cells is shown in Fig. 1a. Positive chymase expression was detected in endothelial cells transfected with pChym but not in control cells transfected with the control vector only. Using this chymase overexpression cell model, the endothelial expression of ACE and chymase was then determined by western blotting. Our results showed that chymase protein expression was detected in cells transfected with chymase gene but much more reduced in control cells. Surprisingly, we found that ACE was strongly expressed in control cells but was significantly reduced in cells transfected with pChym (p < 0.01; Fig. 1b). The bar graph shows the relative density of ACE and chymase expression after normalization with β-actin expression in each sample. These results demonstrated that ACE is likely the dominant angiotensin-generating enzyme in endothelial cells under unstimulated conditions. However, ACE expression was significantly suppressed when chymase was induced in endothelial cells.
Chymase and ACE expression in endothelial cells transfected with or without the chymase gene. a Chymase expression detected by immunofluorescence staining in endothelial cells transfected with the chymase gene. Chymase expression was barely detectable in cells transfected with the control vector. b Expression of chymase and ACE in cells transfected with or without the chymase gene. ACE was strongly expressed in control cells, but ACE expression was downregulated in cells transfected with the chymase gene. In this experiment, cellular protein was collected 24–30 h after pChym transfection. The bar graphs show the relative density of ACE and chymase protein expression after normalization with β-actin expression in each sample. The data are expressed as the means ± SE from five independent transfection experiments. **p < 0.01: cells transfected with the chymase gene vs. control cells
HDAC inhibition promotes chymase activation in endothelial cells
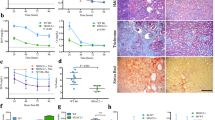
We previously reported that chymase expression is upregulated in vascular endothelium in women with preeclampsia [10, 11]. We also demonstrated that chymase activation is associated with increased inflammatory responses in endothelial cells [16]. However, the mechanism(s) of chymase activation in vascular endothelium is largely unknown. Because the acetylation of histones in chromatin is one of the molecular mechanisms involved in the regulation of gene transcription and is tightly controlled by the balance of acetyltransferase and deacetylase activities, we tested whether HDAC inhibition could modulate chymase expression in endothelial cells. In this experiment, the HDAC inhibitor trichostatin A (TSA) was used, and the chymase and ACE expression levels were then determined. Cells transfected with pChym were treated with TSA at the concentrations of 0, 0.1, 0.3, and 1.0 µM for 24 h. Cells transfected with pZs vector were also treated with TSA and served as controls. Interestingly, we found that chymase expression was dose dependently increased in cells transfected with chymase gene. By comparison, chymase expression was undetectable in control cells. A representative blot of chymase expression in cells with and without pChym transfection is shown in Fig. 2a.
Effects of HDAC inhibition on chymase and ACE expression in endothelial cells with or without transfection of the chymase gene. TSA, an HDAC inhibitor, was used. a Chymase expression was dose dependently increased in cells transfected with the chymase gene when the cells were treated with TSA at the concentrations of 0, 0.1, 0.3, and 1.0 µM. This phenomenon was not seen in cells transfected with the control vector. b TSA induced a dose-dependent increase in chymase expression but a dose-dependent decrease in ACE expression in endothelial cells transfected with the chymase gene. In this experiment, cells were treated with TSA 24 h after cells were transfected with the pChym gene. The treatment was carried out for 24 h, and then total cellular protein was collected. The bar graphs show the relative density of protein expression for chymase and ACE after normalization with β-actin expression in each sample. The ratio of the relative density for chymase to ACE expression was also dose dependently increased. c HDAC1 and HDAC6 expression in TSA-treated cells. The bar graphs show the relative density of HDAC1 and HDAC6 expression after normalization with β-actin expression in each sample. HDAC1 and HDAC6 expression were dose dependently reduced in cells treated with TSA. *p < 0.05 and **p < 0.01: TSA treated vs. not treated. The data are expressed as the means ± SE from four independent experiments
We next determined effect of chymase overexpression on ACE expression in cells treated with TSA. As shown in Fig. 2b, chymase expression was significantly increased in cells treated with TSA. Again, TSA-induced upregulation of chymase expression occurred in a dose-dependent manner. Intriguingly, ACE expression was downregulated in a dose-dependent manner in cells treated with TSA. Similar results were also found in cells treated with the HDAC inhibitor valproic acid (VPA) (data not shown). Thus, the ratio of chymase to ACE expression was significantly increased in cells in which chymase was overexpressed and the increased chymase to ACE ratio was also dose-dependent (Fig. 2b).
The effects of TSA on HDAC1 and HDAC6 expression were examined in cells transfected with the chymase gene. HDAC1 is a member of class I HDACs located in the nucleus, and HDAC6 is a member of class II HDACs mainly located in the cytoplasm. As shown in Fig. 2c, the expression levels of both HDAC1 and HDAC6 were dose dependently reduced in cells treated with TSA. These results indicate that altered HDAC activity/expression could downregulate ACE expression when chymase is activated.
Increased chymase expression is associated with reduced HDAC expression in the maternal vasculature in preeclampsia
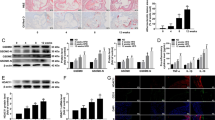
As previously mentioned, chymase expression is increased in maternal vascular endothelium in preeclampsia. To further confirm whether altered HDAC expression is present in the maternal vasculature in preeclampsia, the chymase, ACE, HDAC1, and HDAC6 expression levels were examined by immunostaining in subcutaneous adipose tissue sections from normal and preeclamptic pregnant women. Angiotensin II receptor-1 (AT-1) and HDAC3 expression were also determined. Representative images for chymase, ACE, AT-1, HDAC1, HDAC3, and HDAC6 expression are presented in Fig. 3. Our results showed that chymase expression is markedly increased in maternal vessel endothelium in women with preeclampsia compared with that in normal pregnant controls, a finding that is consistent with our previous observations [10, 11]. ACE expression was not significantly different in vessels between normal and preeclamptic specimen. AT-1 expression was also markedly increased in the smooth muscle layer of vessels from preeclamptic women compared with AT-1 expression in the smooth muscle layer of vessels from normal pregnant women. As expected, the expression levels of HDAC1, HDAC3, and HDAC6 were all markedly reduced in maternal vessels from preeclampsia women compared with normal pregnant controls.
Vascular expression of chymase, ACE, AT-1, HDAC1, HDAC3, and HDAC6 in normotensive and preeclamptic pregnant women. Subcutaneous adipose tissue sections from six normotensive and six preeclamptic pregnancies were examined. Increased endothelial chymase expression was associated with increased AT-1 expression in vascular smooth muscle layers and reduced HDAC expression in systemic vessels from women with preeclampsia (PE) compared with tissue specimens from normotensive (Normal) pregnant controls. Consistent results were obtained. a, b chymase, c, d ACE, e, f AT-1, g, h HDAC1, i, j HDAC3, and k, l HDAC6. a, c, e, g, i, k: Normal; b, d, f, h, j, l PE. Bar = 50 micron
Discussion
Chymase is well-documented as an ACE-independent angiotensin convertase. The function of this serine protease in normal physiology is largely unknown, but chymase activation has been implicated in the pathogenesis of several cardiovascular diseases, including heart failure, cardiac hypertrophy, and diabetes mellitus [17,18,19]. Additionally, numerous studies have demonstrated that chymase participates in vascular remodeling through the degradation of extracellular matrix metalloproteinases (MMPs) and activation of transforming growth factor beta (TGF-β) [20,21,22]. Animal studies have also revealed that chymase contributes to the development of atherosclerosis by eliminating high-density lipoprotein (HDL) and by inhibiting apolipoprotein-dependent removal of cholesterol [23, 24]. Although it was reported that advanced glycation end products (AGEs), markers of oxidative stress, could activate a chymase-dependent angiotensin II-generating pathway in diabetic complications [25], the mechanism of chymase activation in cardiovascular diseases is elusive. In the present study, using chymase overexpression as a test situation, we investigated the potential mechanism(s) of chymase activation in endothelial cells. We specifically tested the role of HDAC inhibition-mediated chymase activation. The consequence of chymase activation on ACE expression was also determined.
Our results showed that ACE is strongly expressed in control endothelial cells, in which chymase is weakly detectable. By contrast, when cells were induced to express chymase, ACE expression was significantly downregulated. These observations suggest that, under normal physiological conditions, ACE is likely the major angiotensin II-generating enzyme in endothelial cells, but this may not be the case when chymase is activated. Presently, we do not understand why the upregulation of chymase expression (chymase transfection) leads to the downregulation of ACE expression. However, since both ACE and chymase convert angiotensin I to angiotensin II, our data suggest that there may be an intrinsic balance between ACE and chymase that controls angiotensin II generation in endothelial cells. Although we did not measure angiotensin II production, our previous studies showed that endothelial angiotensin II production is suppressed by chymase inhibition, but not by ACE inhibition, when chymase is activated [11, 26], indicating that endothelial chymase activation is responsible for increased angiotensin II production.
Epigenetic regulation involving processes, such as DNA methylation and histone modification, are important mechanisms that regulate cellular functions like the stimulation of cell proliferation, alteration of gene/protein activity and expression, and induction of phenotypic changes. The reason for the chymase overexpression-mediated downregulation of ACE expression is unknown. To study whether epigenetic regulation participates in the regulation of chymase and ACE expression in endothelial cells, the HDAC inhibitor TSA was used. Surprisingly, we found that, in cells induced with chymase, chymase expression was dose dependently increased when cells were exposed to the HDAC inhibitor TSA (Fig. 2). Most interestingly, ACE expression was dose dependently decreased in cells treated with TSA. Similar results were also seen in cells treated with the HDAC inhibitor VPA (data not shown)—i.e., chymase expression was dose dependently increased, and ACE expression was dose dependently decreased in chymase-overexpressed cells when they were treated with HDAC inhibitors. Thus, the ratio of chymase to ACE expression was significantly increased or vice versa. These results suggest that chymase may represent the major angiotensin II-producing enzyme when chymase is activated in endothelial cells [11]. Although there was a discrepancy in chymase and ACE expression shown in Fig. 1b and Fig. 2b in cells transfected with pChym, our results of TSA-induced upregulation of chymase expression and the increased ratio of chymase to ACE expression in cells transfected with the chymase gene are consistent with our observed increased chymase expression and reduced expression of HDACs in maternal vessels from women with preeclampsia.
TSA is an HDAC inhibitor that inhibits both class I and class II HDACs but not class III HDACs (i.e., sirtuins). The demonstration of HDAC inhibition was confirmed by the detection of HDAC1 and HDAC6 expression. Our results showed that reduced HDAC1 and HDAC6 protein expression is also dose-dependent. Additionally, we found that acetylated histone proteins were accumulated in cells treated with TSA, and the response was dose-dependent (data not shown), indicating that HDAC activities are inhibited in cells treated with TSA. Although we do not know how TSA suppresses HDAC1 and HDAC6 protein expression, a similar phenomenon that HDAC inhibitors (MHA219 and SAHA) suppressed HDAC protein expression accompanied with increased acetylated histone protein expression was reported in DU145, LNCap, and PC3 cell lines [27]. In the present study, we found that the upregulation of chymase expression related to the reduced HDAC expression seen in maternal vessels from women with preeclampsia is consistent with the results of the downregulation of HDAC1 and HDAC6 expression in endothelial cells treated with the HDAC inhibitor. Presently, we do not know whether the changes in HDAC expression are physiologically effective in vivo, and we do not know which HDAC(s) regulate chymase expression. However, the observation of the increased acetylated histone H3 and H4 protein accumulation associated with chymase activation when TSA is used in chymase-overexpressed cells suggest the association of aberrant HDAC activity and chymase activation in endothelial cells.
Although no artery function experiment was performed in the present study, the work reported by Uehara et al. demonstrated the importance of chymase-mediated angiotensin II generation in human vascular tissue [28]. Using human internal thoracic artery homogenates from patients with hypercholesterolemia, Uehara et al. found that the formation of angiotensin II was reduced by 95% when chymase inhibitor chymostatin was used [28]. Similar results were also obtained by Takai et al. in which, using samples from human gastroepiploic arteries, they found that angiotensin II formation was inhibited by 8% with the ACE inhibitor lisinopril but by 95% with chymostatin [29]. Takai et al. also compared ACE and chymase-mediated angiotensin II formation in tissue slices of human gastroepiploic arteries [30] and found that angiotensin II formation was reduced by 5% with lisinopril but was reduced by 90% when chymostatin was used [30]. These findings are important regarding the consequences of chymase activation and the role of chymase in angiotensin II generation in the systemic vasculature. Recently, chymase-dependent production of angiotensin II was also reported in aged hearts in an animal study [31]. Although the report of chymase-mediated angiotensin II generation in the vasculature under in vivo conditions is lacking, the results from these ex vivo studies with vessel homogenates and slices as well as in vitro cell culture and animal studies provide considerable evidence to support the notion that chymase plays a key role in angiotensin II formation in the cardiovascular system when it is activated.
We previously reported increased chymase expression in the maternal vascular endothelium from women with preeclampsia [10, 11]. To determine whether altered HDAC expression is also present in the systemic vasculature in women with preeclampsia, we examined chymase, ACE, HDAC1, HDAC3, and HDAC6 expression in subcutaneous adipose tissue sections from normotensive and preeclamptic pregnant women. Consistent with our previous findings, chymase expression was found to be markedly increased in the maternal vascular endothelium from preeclampsia compared with those from normal pregnant controls. Although immunostaining for ACE expression in maternal vessel endothelium showed no difference between the normal and preeclamptic groups among our study subjects, noticeably increased chymase over ACE expression was seen in the maternal vessel endothelium from preeclampsia, a finding that is consistent with the observation of dominant chymase expression over ACE expression in chymase-overexpressed cells when HDACs are inhibited. We understand there are limitations in our in vitro cell model to explore the underlying mechanisms chymase activation in preeclampsia—i.e., results obtained from in vitro studies could not completely recapture the maternal systemic vascular changes in preeclampsia. Moreover, it is impossible to test the consequences of HDAC inhibition-induced aberrant chymase and ACE expression under in vivo condition in humans during pregnancy. However, the phenomenon of dominant chymase expression over ACE expression associated with reduced HDAC expression in the maternal vasculature suggests that imbalanced chymase and ACE expression is related to aberrant HDAC activity in the systemic vasculature in women with preeclampsia compared with normotensive pregnant controls.
The HDAC family has 11 members. We examined the expression of three major HDACs: HDAC1, HDAC3, and HDAC6. HDAC1 and 3 belong to class I HDACs and are located in the nucleus. HDAC6 belongs to class II HDACs and is located mainly in the cytoplasm. Strikingly, the expression levels of HDAC1, HDAC3, and HDAC6 were all reduced in the maternal vasculature in preeclampsia women compared with that in normal pregnant women. Although the sample size was small in the present study, using a similar sample size, we previously demonstrated that the downregulation of anti-inflammatory mediator suppressor of cytokine signaling-3 expression, upregulation of inflammatory microRNA-203 expression and inflammatory cytokine IL-16 expression in the maternal vessel endothelium in preeclampsia compared with those in normal pregnant controls [32,33,34]. Additionally, we examined AT-1 expression in the same set of subcutaneous adipose tissues from normal and preeclamptic pregnancies. Our results showed that AT-1 expression is strongly expressed in the maternal vessel smooth muscle layers in preeclampsia compared with that in normal pregnant controls. It should be noted that the representative imaging of the vascular expression of chymase, ACE, AT-1, and HDACs shown in Fig. 3 were all resistance arteries in the range of 100–300 microns in diameter. Although the vascular responses to vasoactivators could differ between arteries and veins with vessel size and location, our findings of increased endothelial chymase expression and increased AT-1 expression in vascular smooth muscle cells combined with reduced HDAC expression in the maternal resistance vessels in preeclampsia provide compelling evidence that the upregulation of chymase expression is associated with increased vascular resistance in preeclampsia.
Furthermore, our observations that the upregulation of chymase resulted in the downregulation of ACE expression in endothelial cells provides powerful evidence, which may explain the source of angiotensin II in the circulation in the so-called “ACE-escape” phenomenon, a clinical situation in which the plasma levels of angiotensin II rise above basal values or gradually return to the pretreatment values in patients after the long-term use of ACE inhibitors [35, 36]. The “ACE-escape” phenomenon is also the rationale for dual RAS blockade of ACE inhibitors (ACEIs) and angiotensin receptor AT-1 blockers (ARBs), which lead to the synergistic blockade of RAS not obtainable by the administration of either drug alone. Moreover, it is also expected that chymase will represent a significant therapeutic target to treat cardiovascular diseases [2].
In addition to angiotensin II-generating activity, chymase is also considered an inflammatory protease with many potent pro-inflammatory properties [20]. Mast cell chymase can induce the accumulation of neutrophils, eosinophils, and other inflammatory cells in inflamed tissues [22]. Animal studies have revealed that specific chymase inhibition was able to suppress neutrophil accumulation in the lung and to reduce silica-induced pulmonary fibrosis (in mice) [37]. Chymase could also trigger the production of cytokines and chemokines to indirectly stimulate the infiltration of inflammatory cells. In mast cells, degranulation not only releases chymase but also biogenic amines, TNFα, serglycin proteoglycans, and various lysosomal enzymes [38]. Moreover, increased chymase expression was found to be temporally associated with increased endothelial inflammatory responses in preeclampsia, as evidenced by increased endothelial P-selectin and E-selectin expression [39] and decreased anti-inflammatory mediator suppressor of cytokine signaling-3 expression in the maternal systemic vasculature [32]. All these findings indicate that chymase activation not only contributes to vasoconstriction via the generation of angiotensin II but also potentially promotes inflammatory responses via the production of inflammatory mediators and cytokines in the vasculature. Therefore, chymase activation could represent a significant and important contributor to vascular dysfunction in preeclampsia and other cardiovascular disorders.
In conclusion, this is the first study to show that HDAC inhibition can trigger chymase activation and inhibit ACE expression, consequently perturbing the balance between ACE and chymase in endothelial cells when chymase is activated. Increased chymase and AT-1 expression was related to reduced HDAC expression in maternal-resistant vessels in preeclampsia compared with that in normal pregnant controls. These results are highly meaningful mechanistically and suggest that chymase may be the major angiotensin II generating enzyme once this serine protease is activated. In addition, a balance between chymase and ACE activity in endothelial cells could be the key feature to determine which is the dominant angiotensin II-generating enzyme in the vasculature. Therefore, chymase activation in vascular tissues represents an underlying pathophysiological event in hypertension-related diseases, supporting the clinical importance of chymase as a new pharmacological target for the treatment of cardiovascular disorders.
References
Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–19.
Bacani C, Frishman WH. Chymase: a new pharmacologic target in cardiovascular disease. Cardiol Rev. 2006;14:187–93.
Kelley JL, Chi DS, Abou-Auda W, Smith JK, Krishnaswamy G. The molecular role of mast cells in atherosclerotic cardiovascular disease. Mol Med Today. 2000;6:304–8.
Cristovam PC, Carmona AK, Arnoni CP, Maquigussa E, Pereira LG, Boim MA. Role of chymase in diabetic nephropathy. Exp Biol Med (Maywood). 2012;237:985–92.
Wu Q, Kuo HC, Deng GG. Serine proteases and cardiac function. Biochim Biophys Acta. 2005;1751:82–94.
Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–57.
Richard V, Hurel-Merle S, Scalbert E, Ferry G, Lallemand F, Bessou JP et al. Functional evidence for a role of vascular chymase in the production of angiotensin II in isolated human arteries. Circulation. 2001;104:750–2.
Nakano A, Kishi F, Minami K, Wakabayashi H, Nakaya Y, Kido H. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31-amino acid-length endothelins by chymase from human mast cells. J Immunol. 1997;159:1987–92.
Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M et al. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399–405.
Wang Y, Gu Y, Lewis DF, Alexander JS, Granger DN. Elevated plasma chymotrypsin-like protease (chymase) activity in women with preeclampsia. Hypertens Pregn. 2010;29:253–61.
Gu Y, Lewis DF, Alexander JS, Wang Y. Upregulation of cathepsin C expression contributes to endothelial chymase activation in preeclampsia. Hypertens Res. 2017;40:976–81.
Wang T, Han SX, Zhang SF, Ning YY, Chen L, Chen YJ et al. Role of chymase in cigarette smoke-induced pulmonary artery remodeling and pulmonary hypertension in hamsters. Respir Res. 2010;11:36
Furubayashi K, Takai S, Jin D, Muramatsu M, Ibaraki T, Nishimoto M et al. The significance of chymase in the progression of abdominal aortic aneurysms in dogs. Hypertens Res. 2016;30:349–57.
Devarajan S, Yahiro E, Uehara Y, Habe S, Nishiyama A, Miura S et al. Depressor effect of chymase inhibitor in mice with high salt-induced moderate hypertension. Am J Physiol Heart Circ Physiol. 2015;309:H1987–H1996.
Wang Y, Adair CD, Coe L, Weeks JW, Lewis DF, Alexander JS. Activation of endothelial cells in preeclampsia: increased neutrophil-endothelial adhesion correlates with up-regulation of adhesion molecule P-selectin in human umbilical vein endothelial cells isolated from preeclampsia. J Soc Gynecol Investig. 1998;5:237–43.
Gu Y, Liu C, Alexander JS, Groome LJ, Wang Y. Chymotrypsin-like protease (chymase) mediates endothelial activation by factors derived from preeclamptic placentas. Reprod Sci. 2009;16:905–13.
Batlle M, Roig E, Perez-Villa F, Lario S, Cejudo-Martin P, Garcia-Pras E et al. Increased expression of the renin-angiotensin system and mast cell density but not of angiotensin-converting enzyme II in late stages of human heart failure. J Heart Lung Transplant. 2006;25:1117–25.
Huang XR, Chen WY, Truong LD, Lan HY. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol. 2003;14:1738–47.
Pfeufer A, Osterziel KJ, Urata H, Borck G, Schuster H, Wienker T et al. Angiotensin-converting enzyme and heart chymase gene polymorphisms in hypertrophic cardiomyopathy. Am J Cardiol. 1996;78:362–4.
Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174:821–5.
Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689–96.
Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–6.
Lee M, Sommerhoff CP, von Eckardstein A, Zettl F, Fritz H, Kovanen PT. Mast cell tryptase degrades HDL and blocks its function as an acceptor of cellular cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:2086–91.
Lee M, Calabresi L, Chiesa G, Franceschini G, Kovanen PT. Mast cell chymase degrades apoE and apoA-II in apoA-I-knockout mouse plasma and reduces its ability to promote cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2002;22:1475–81.
Koka V, Wang W, Huang XR, Kim-Mitsuyama S, Truong LD, Lan HY. Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation. 2006;113:1353–60.
Wang Y, Gu Y, Lewis DF. Endothelial angiotensin II generation induced by placenta-derived factors from preeclampsia. Reprod Sci. 2008;15:932–8.
Patra N, De U, Kim TH, Lee YJ, Ahn MY, Kim ND et al. A novel histone deacetylase (HDAC) inhibitor MHY219 induces apoptosis via up-regulation of androgen receptor expression in human prostate cancer cells. Biomed Pharmacother. 2013;67:407–15.
Uehara Y, Urata H, Sasaguri M, Ideishi M, Sakata N, Tashiro T et al. Increased chymase activity in internal thoracic artery of patients with hypercholesterolemia. Hypertension. 2000;35(1 Pt 1):55–60.
Takai S, Sakaguchi M, Jin D, Yamada M, Kirimura K, Miyazaki M. Different angiotensin II-forming pathways in human and rat vascular tissues. Clin Chim Acta. 2001;305:191–5.
Takai S, Shiota N, Jin D, Miyazaki M. Functional role of chymase in angiotensin II formation in human vascular tissue. J Cardiovasc Pharmacol. 1998;32:826–33.
Froogh G, Pinto JT, Le Y, Kandhi S, Aleligne Y, Huang A et al. Chymase-dependent production of angiotensin II: an old enzyme in old hearts. Am J Physiol Heart Circ Physiol. 2017;312:H223–H231.
Wang Y, Lewis DF, Gu Y, Zhao S, Groome LJ. Elevated maternal soluble gp130 and IL-6 levels and reduced gp130 and SOCS-3 expressions in women with preeclampsia. Hypertension. 2011;57:336–42.
Wang Y, Dong Q, Gu Y, Groome LJ. Up-regulation of miR-203 expression induces endothelial inflammatory response: potential role in preeclampsia. Am J Reprod Immunol. 2016;76:482–90.
Gu Y, Lewis DF, Deere K, Groome LJ, Wang Y. Elevated maternal IL-16 levels, enhanced IL-16 expressions in endothelium and leukocytes, and increased IL-16 production by placental trophoblasts in women with preeclampsia. J Immunol. 2008;181:4418–22.
van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ et al. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106:367–72.
Chrysant SG. Current status of dual renin angiotensin aldosterone system blockade for the treatment of cardiovascular diseases. Am J Cardiol. 2010;105:849–52.
Takato H, Yasui M, Ichikawa Y, Waseda Y, Inuzuka K, Nishizawa Y et al. The specific chymase inhibitor TY-51469 suppresses the accumulation of neutrophils in the lung and reduces silica-induced pulmonary fibrosis in mice. Exp Lung Res. 2011;37:101–8.
Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases- multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–90.
Wang Y, Zhang Y, Lewis DF, Gu Y, Li H, Granger DN et al. Protease chymotrypsin mediates the endothelial expression of P- and E-selectin, but not ICAM and VCAM, induced by placental trophoblasts from preeclamptic pregnancies. Placenta. 2003;24:851–61.
Acknowledgements
This study was supported by a grant from the National Institutes of Health NICHD R21HD076289 to Yuping Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Y., Gu, Y., Alexander, J.S. et al. Histone deacetylase inhibition disturbs the balance between ACE and chymase expression in endothelial cells: a potential mechanism of chymase activation in preeclampsia. Hypertens Res 42, 155–164 (2019). https://doi.org/10.1038/s41440-018-0150-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0150-1
Keywords
This article is cited by
-
Deciphering the Epigenetic Landscape: Placental Development and Its Role in Pregnancy Outcomes
Stem Cell Reviews and Reports (2024)
-
MiR-222-3p Inhibits Trophoblast Cell Migration and Alleviates Preeclampsia in Rats Through Inhibiting HDAC6 and Notch1 Signaling
Reproductive Sciences (2022)
-
HDAC5 inactivates CYR61-regulated CD31/mTOR axis to prevent the occurrence of preeclampsia
Cell and Tissue Research (2022)