Abstract
We evaluated the relationship between blood pressure variability (BPV) and the development of hypertension during pregnancy. A total of 4163 pregnant women with normal blood pressure (BP) before 20 weeks of gestation were included in this study. The visit-to-visit blood pressure variability (VVV) was evaluated using the standard deviation (SD) of the systolic BP taken three times during pregnancy at approximately 10, 20, and 30 weeks of gestation. The VVV gradually decreased during pregnancy in normotensive subjects (SD: 7.2 ± 4.2 mmHg, 6.8 ± 3.9 mmHg, and 6.3 ± 3.6 mmHg at 10, 20, and 30 weeks, respectively). However, the VVV of hypertensive subjects did not decrease (SD: 8.2 ± 5.7 mmHg, 7.6 ± 5.0 mmHg, and 8.3 ± 5.3 mmHg at 10, 20, and 30 weeks, respectively) and was significantly greater than the VVV of normotensive subjects (p < 0.001). The VVV was significantly higher in patients who developed hypertension, and there was no decrease in VVV during pregnancy. Pregnancy complications were significantly increased in women with higher VVV at 10 and 30 weeks. Therefore, increased VVV during pregnancy may be a predictor of poor pregnancy outcomes.
Similar content being viewed by others
Introduction
Visit-to-visit blood pressure variability (VVV) is considered an important cardiovascular risk factor, and evidence has shown that the VVV is closely related to hypertension-associated end organ damage, cardiovascular events, and mortality [1]. The relationship may be explained by not only the treatment-related factors [2] but also the spontaneous variation in biological and behavioral factors [3]. Age, sex, higher systolic blood pressure (BP) and pulse pressure, and a history of a cardiovascular event increase the VVV [4]. However, such results originate from studies performed in hypertensive patients. The relationship between VVV and clinical outcomes has not been precisely evaluated in pregnant women whose hemodynamic changes are unique [5].
During pregnancy, profound changes occur in the maternal hemodynamic system. Cardiac output and heart rate increase, and systemic vascular resistance decreases. Blood volume then begins to increase [6, 7]. The response of the autonomic nervous system, which also changes across gestation, further influences the maternal hemodynamic system. With such changes, blood pressure (BP) decreases until 20 weeks of gestation and then increases until the end of pregnancy [8]. Therefore, BP variability is expected to show a unique pattern during pregnancy and to have a clinical impact on pregnancy outcomes. However, BP variability during pregnancy has not been serially assessed, and only limited data on short-term BP variability without serial evaluation have been reported for pregnant women [9,10,11].
This study evaluated the blood pressure variability in pregnant women during three trimesters and the pregnancy outcomes. We compared the VVV during pregnancy and compared its characteristics between hypertensive and normotensive women during pregnancy and its association with pregnancy outcomes.
Methods
Study population
We retrospectively investigated pregnant women who visited Cheil General Hospital and Women’s Healthcare Center before 10 weeks of gestation and received antenatal care until delivery between January 1, 2001, and December 31, 2010. We included patients who were diagnosed with hypertension after 20 weeks of gestation and who had sufficient BP data to evaluate VVV at three time points at approximately 10, 20, and 30 weeks of gestation. Patients were excluded if they were diagnosed with hypertension before 20 weeks, experienced proteinuria before 20 weeks, or had other pregnancy-related complications that could influence pregnancy outcomes. For the control group, normotensive pregnant subjects with sufficient BP data for VVV evaluation who received antenatal care at the same hospital from January 1, 2008, to December 31, 2008, were included (Fig. 1). Approval for this study was obtained from the Institutional Review Board of Cheil General Hospital (IRB number CGH–IRB-2011-79).
Data acquisition
Patient data including BP, age, body mass index before pregnancy, body weight change during pregnancy, past obstetric history, presence or absence of proteinuria at first prenatal visit, other previous medical history, and maternal and neonatal outcomes of the pregnancy were collected from medical records. The BP during antenatal visits was measured using an automated oscillometric device in a sitting position after at least 5 min of rest. Hypertension was diagnosed as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mm Hg with (pre-eclampsia) or without (gestational hypertension) proteinuria of ≥1+ by dipstick test or ≥300 mg/day by 24-h urine study, kidney or liver dysfunction, thrombocytopenia or edema [12]. We recorded the delivery methods and the development of placental abruptio, preterm labor, pulmonary edema, deep vein thrombosis, pulmonary thromboembolism, hemolysis, elevated liver enymes, and low platelets syndrome, heart failure, and maternal death to evaluate maternal complications [13]. To evaluate fetal complications, we recorded 1-min and 5-min Apgar scores, birth weight, the presence of meconium in the amniotic fluid, respiratory distress syndrome, and fetal death.
Blood pressure variability
The VVV was evaluated three times at approximately 10, 20, and 30 weeks of gestation. To acquire the VVV, three BP measurements obtained on different visits at each of the time points were used if the interval between visits was less than 2 weeks. The VVV was determined by calculating the standard deviation (SD) of systolic BPs. Considering the possible influence of absolute BP values on the VVV, the coefficient of variation (CV) was calculated using the following formula: SD/mean systolic BP at each week × 100 (%) (Fig. 1).
Statistical analysis
Data are expressed as the means ± standard deviation (SD) for continuous variables and the number (%) for categorical variables. The differences between groups for the continuous variables were compared using the independent t-test. To control for different conditions in the normotensive and hypertensive subjects, 1:1 propensity score matching analysis was used. We estimated the propensity score for each patient using a logistic regression model that included the following variables: age, body mass index, weight gain, parity, and presence of multiple pregnancies. The propensity score model was well calibrated (Hosmer–Lemeshow goodness-of-fit test, p = 0.168) with good discrimination (c-statistic = 0.754). The differences between groups in the categorical variables were compared using chi-square tests. The changes in BP and VVV at 10, 20, and 30 weeks of gestation were compared using repeated measures analysis of variance. The differences in VVV between normotensive and hypertensive subjects at each time point were analyzed using the independent t-test. A logistic regression analysis was performed to examine the association between the VVV divided by its median value at each time point and pregnancy outcome. The least square (LS) means of the VVV were compared between the presence and absence of maternal and fetal pregnancy complications at each time point of the pregnancy after adjusting for age, body weight before pregnancy, weight gain during pregnancy, and mean systolic blood pressure for each gestational age in our logistic regression model. A p-value < 0.05 was considered statistically significant. For database management and statistical analysis, we used Statistical Analysis System (SAS) software, version 9.3 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Among the 2231 hypertensive subjects who received antenatal care at our center during the study period, 484 patients had sufficient BP data to evaluate the VVV at all three time points. For the control group, of the 6700 normotensive subjects identified in our initial review of records, 3679 patients were included. They were 32.6 ± 3.5 years of age and weighed 55.2 ± 8.2 kg before pregnancy. The body mass index before pregnancy was 21.2 ± 3.0 m/kg2.
The mean gestational age at hypertension onset was 34.5 ± 6.9 weeks. The subjects with hypertension were younger than the normotensive subjects (p < 0.001); however, the hypertensive patients had higher values of body weight (p < 0.001), body mass index before pregnancy (p < 0.001), and weight gain during pregnancy (p < 0.001). The gestational age at delivery was 39.1 ± 1.4 weeks in all patients. However, the gestational age was 39.3 ± 1.3 weeks in normotensive women and 38.1 ± 1.9 weeks in hypertensive patients (p < 0.001). Development of complications during pregnancy was more frequent in hypertensive patients (p < 0.001). These differences were adjusted using 1:1 propensity matching considering age, height, body mass index, weight gain, parity, and presence of multiple pregnancies to evaluate the differences in systolic and diastolic blood pressure and the VVV between patients with and without developing hypertension during pregnancy (Table 1).
Blood pressure change during pregnancy
At 10 weeks, the systolic BP was 114.3 ± 10.3 mmHg, and the diastolic BP was 66.2 ± 7.7 mmHg. At 20 weeks, the systolic and diastolic BPs were 113.1 ± 10.2 mmHg and 64.8 ± 7.3 mmHg, respectively; at 30 weeks, the systolic and diastolic BPs were 115.4 ± 10.4 mmHg and 67.2 ± 7.7 mmHg, respectively, in normotensive women. The BP significantly decreased at 20 weeks and then increased at 30 weeks. However, in hypertensive subjects, decreases in systolic and diastolic BPs at approximately 20 weeks were blunted, and the increase in BP at 30 weeks was augmented. The systolic and diastolic BPs were significantly higher in the hypertensive subjects than in the normotensive subjects throughout pregnancy even during the period before the development of hypertension (p < 0.001 in systole and diastole at 10, 20, and 30 weeks), and these differences were still significant after the 1:1 propensity score matching adjustment for differences in baseline characteristics between groups at all periods (Table 1, Fig. 2).
Visit-to-visit blood pressure variability changes during pregnancy
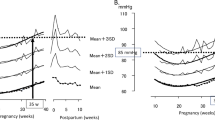
Irrespective of absolute BP values, VVV parameters, SD and CV gradually decreased for the normotensive subjects (SD, 7.2 ± 4.2 mmHg, 6.8 ± 3.9 mmHg, 6.3 ± 3.4 mmHg, p < 0.001 at 10, 20, and 30 weeks, respectively; CV, 6.4 ± 3.8%, 6.0 ± 3.5%, 5.5 ± 3.2%, p < 0.001 at 10, 20, and 30 weeks, respectively), and these reductions were still significant after adjusting for systolic BP. In contrast, the VVV was not changed throughout pregnancy in the hypertensive subjects (SD, 8.2 ± 5.7 mmHg, 7.6 ± 5.0 mmHg, 8.3 ± 5.3 mmHg, p = 0.062 at 10, 20, and 30 weeks, respectively; CV, 6.7 ± 4.4%, 6.3 ± 4.2%, 6.5 ± 4.0%, p = 0.278 at 10, 20, and 30 weeks, respectively). Pattern changes in VVV between normotensive and hypertensive subjects were significantly different (p < 0.001 for SD and p = 0.001 for CV). Additionally, VVV presented as the SD was significantly higher in the hypertensive patients than in the normotensive subjects throughout pregnancy (p < 0.001 for all periods), and VVV presented as the CV was significantly higher at 30 weeks (p = 0.170, p = 0.242, and p < 0.001 at 10, 20, and 30 weeks of gestation, respectively; Fig. 3). The difference in VVV between normotensive and hypertensive subjects at 30 weeks was still significant after adjusting for age, body weight before pregnancy, weight gain during pregnancy, and systolic BP (p < 0.001 for both the SD and CV). Moreover, propensity score matching did not change the difference in VVV between normotensive and hypertensive subjects (Table 2).
Visit-to-visit blood pressure variability and pregnancy outcomes
Finally, we evaluated the influence of VVV at each time point on pregnancy outcome. We divided all subjects into 2 groups by the VVV (the median value), and the development of maternal and fetal complications was compared between groups. We found that the patient group with increased VVV at 10 weeks and 30 weeks of gestation showed significantly worse pregnancy outcomes. In patients with a VVV (by SD) over 5.86 mmHg at 30 weeks, maternal and/or fetal complications occurred in 346 (16.8%) patients; for patients with a VVV under 5.86 mmHg (by SD), there were 287 (13.7%) patients with complications. Patients with a VVV over 5.86 were more likely to have maternal and/or fetal complications (OR, 1.25; 95% confidence interval, 1.05–1.49; p = 0.012) (Table 3). The influence was much greater when the influence of the VVV was evaluated only among hypertensive patients with an odds ratio of 1.53 (95% confidence interval, 1.04–2.26; p = 0.031 by SD at 30 weeks of gestation). The VVV was also significantly greater in the group of patients with maternal and/or fetal complications (Fig. 4).
Blood pressure variability difference due to the presence of maternal and fetal pregnancy complications in each period of pregnancy; the patient age, body weight before pregnancy, weight gain during pregnancy and mean systolic BP at each approximate gestational age were adjusted. SD standard deviation (mmHg), CV coefficient of variation (%)
Discussion
We evaluated the characteristics of VVV during pregnancy and investigated the difference in VVV between normotensive and hypertensive patients during pregnancy and the relationship between VVV and pregnancy complications. The VVV gradually decreased in the normotensive women during pregnancy; however, the VVV did not change significantly in the subjects with hypertension. The VVV was significantly higher in the hypertensive pregnant patients than in the normotensive pregnant subjects. The pregnancy outcomes were also worse in patients with increased VVV.
Maternal hemodynamics begin to change within 5 weeks of gestation. First, heart rate increases from 5 weeks of gestation. Systemic vascular resistance decreases at 5 weeks and reaches its peak level at approximately 20 weeks [14], which causes an increase in stroke volume. The blood volume also increases from 4 weeks to 34 weeks of gestation. With these changes, cardiac output increases up to 45% until 24 weeks of gestation and then reaches a plateau [7, 15, 16]; this increase in cardiac output is accompanied by structural changes including enlargements of the left ventricle and its apparatus [6]. These changes may influence the absolute BP value and BP variability during pregnancy. In our study, the VVV gradually decreased throughout pregnancy in patients with normal BP irrespective of the absolute BP value. This downward trend may be associated with changes in hemodynamic parameters that begin at approximately 5 weeks of gestation, reach their peak in the second trimester, and then achieve a static state.
Hypertension in pregnancy differs from primary hypertension and has its own pathophysiology [5]. Although metabolic factors such as hyperlipidemia and insulin resistance contribute to endothelial dysfunction and influence the development of pre-eclampsia [17, 18], the main pathognomonic factors of hypertension in pregnancy are believed to be cardiovascular maladaptation and vasoconstriction due to abnormal placental vasculature. Placental under perfusion causes the release of antiangiogenic factors and other substances that can cause maternal endothelial dysfunction [19]. Sympathetic overactivity in pre-eclampsia may also influence hypertension and hemodynamic instability. Such changes may also influence VVV. Therefore, as BP increased before the diagnosis of hypertension, VVV was increased in the patient group who finally developed hypertension in which the association was weak but significant. However, it is not clear whether VVV may affect the development of hypertension or if hypertension development may further affect the increase in VVV.
Many studies evaluating BP variability in hypertensive patients have concluded that increased BP variability contributes to adverse cardiovascular outcomes of hypertension independent of absolute BP values [1]. The development of cardiovascular damage due to hypertension and cardiovascular mortality has been significantly related to increased short-term and long-term BP variability [20, 21]. In the case of short-term BPV, increased central sympathetic drive, reduced cardiopulmonary reflex [22], humoral factors [23, 24] and behavioral influence are considered to contribute to its increase. In the case of long-term BP variability, although its pathophysiology has not been well elucidated [25], increased arterial stiffness has been proposed as one cause of increased long-term BP variability among treated hypertensive patients with poorly controlled BP [2].
In this study, we evaluated the VVV with relatively short intervals of less than 2 weeks, which is much shorter than visit intervals for nonpregnant patients with hypertension. Considering that the hemodynamic condition continuously changes during pregnancy [8], the VVV at each time point in this study may reflect the degree of BP oscillations at different periods of gestation with gradual changes in vascular resistance, heart rate, and blood volume. The significant difference in VVV due to the presence of hypertension and poor pregnancy outcome in this study may also be related to endothelial dysfunction or sympathetic overactivity, whose pathophysiology is similar to that of nonpregnant patients with hypertension. However, neither drug adherence nor poor blood pressure control is indicated as a reason for increased VVV in the pregnant subjects in this study because most patients were diagnosed with hypertension at approximately 34 weeks of gestation, which is after the evaluation of the VVV at approximately 30 weeks. Moreover, there was no significant difference in VVV between our patients who received antihypertensive medications during pregnancy and those who did not. We may have obtained more concrete results if we performed 24-hour ambulatory BP monitoring at each time point or evaluated the day-to-day BP variability using home BP data [26]. However, it is very difficult to perform ambulatory BP monitoring three times during pregnancy in real life; therefore, the longitudinal evaluation of short-term VVV during pregnancy may be a valuable alternative.
This study has several limitations. First, we have no reference value for the VVV in pregnant women as this is the first known study to evaluate visit-to-visit BPV in pregnant women. The BPV values in this study were not very different from those of nonpregnant individuals reported in previous studies. However, the BPV values for women of the same ages as those in our study were not available. Second, this is a retrospective study. Patients were not regularly followed for BP measurements, and there were variations in the interval between visits for each time point and between patients. To minimize this variability, we selected patients who performed BP evaluation with intervals shorter than 2 weeks for each BPV evaluation. Third, this was a single-center observational study performed in a hospital that specialized in obstetrics and gynecology, where the proportion of high-risk pregnant women is higher than that of the general population; this is the reason for the higher mean age of the normotensive subjects compared to the mean age of the hypertensive patients in this study. The values of other parameters may not accurately represent those of the general population.
Conclusion
VVV gradually decreased in the normotensive pregnant women, whereas VVV in hypertensive patients did not change during pregnancy and was greater than that of the normotensive pregnant women. Additionally, VVV significantly influenced pregnancy outcomes, especially in the hypertensive patients. Therefore, serial assessment of the VVV during pregnancy may be helpful for predicting pregnancy outcomes.
References
Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–55.
Muntner P, Levitan EB, Joyce C, Holt E, Mann D, Oparil S, et al. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. J Clin Hypertens. 2013;15:112–7.
Nagai M, Hoshide S, Nishikawa M, Shimada K, Kario K. Sleep duration and insomnia in the elderly: associations with blood pressure variability and carotid artery remodeling. Am J Hypertens. 2013;26:981–9.
Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–6.
Ekholm EM, Erkkola RU. Autonomic cardiovascular control in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:29–36.
Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. Br Heart J. 1992;68:540–3.
Duvekot JJ, Peeters LL. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv. 1994;49:S1–14.
Hermida RC, Ayala DE, Iglesias M. Predictable blood pressure variability in healthy and complicated pregnancies. Hypertension. 2001;38:736–41.
Voss A, Malberg H, Schumann A, Wessel N, Walther T, Stepan H, et al. Baroreflex sensitivity, heart rate, and blood pressure variability in normal pregnancy. Am J Hypertens. 2000;13:1218–25.
Faber R, Baumert M, Stepan H, Wessel N, Voss A, Walther T. Baroreflex sensitivity, heart rate, and blood pressure variability in hypertensive pregnancy disorders. J Hum Hypertens. 2004;18:707–12.
Flood P, McKinley P, Monk C, Muntner P, Colantonio LD, Goetzl L, et al. Beat-to-beat heart rate and blood pressure variability and hypertensive disease in pregnancy. Am J Perinatol. 2015;32:1050–8.
Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-Induced hypertension. Horm (Athens). 2015;14:211–23.
Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011.
Iizuka M, Miyasaka N, Hirose Y, Toba M, Sakamoto S, Kubota T. Is there a differential impact of parity on factors regulating maternal peripheral resistance? Hypertens Res. 2016;39:737–43.
Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–8.
Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060–1065.
Spracklen CN, Smith CJ, Saftlas AF, Robinson JG, Ryckman KK. Maternal hyperlipidemia and the risk of preeclampsia: a meta-analysis. Am J Epidemiol. 2014;180:346–58.
Abhari FR, Ghanbari Andarieh M, Farokhfar A, Ahmady S. Estimating rate of insulin resistance in patients with preeclampsia using HOMA-IR index and comparison with nonpreeclampsia pregnant women. Biomed Res Int. 2014;2014:140851.
Santillan MK, Santillan DA, Sigmund CD, Hunter SK. From molecules to medicine: a future cure for preeclampsia? Drug News Perspect. 2009;22:531–41.
Parati G, Ochoa JE, Lombardi C, Bilo G. Blood pressure variability: assessment, predictive value, and potential as a therapeutic target. Curr Hypertens Rep. 2015;17:537.
Madden JM, O’Flynn AM, Fitzgerald AP, Kearney PM. Correlation between short-term blood pressure variability and left-ventricular mass index: a meta-analysis. Hypertens Res. 2016;39:171–7.
Parati G, Bilo G. Arterial baroreflex modulation of sympathetic activity and arterial wall properties: new evidence. Hypertension. 2012;59:5–7.
Conway J, Boon N, Davies C, Jones JV, Sleight P. Neural and humoral mechanisms involved in blood pressure variability. J Hypertens. 1984;2:203–8.
Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, et al. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension. 2012;60:369–77.
Mancia G, Messerli F, Bakris G, Zhou Q, Champion A, Pepine CJ. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension. 2007;50:299–305.
Metoki H, Iwama N, Ishikuro M, Satoh M, Murakami T, Nishigori H. Monitoring and evaluation of out-of-office blood pressure during pregnancy. Hypertens Res. 2017;40:107–9.
Acknowledgements
The data were collected by the authors at Cheil General Hospital, Dankook University College of Medicine, Seoul, Korea (South).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, SA., Lee, JD. & Park, J.B. Differences in visit-to-visit blood pressure variability between normotensive and hypertensive pregnant women. Hypertens Res 42, 67–74 (2019). https://doi.org/10.1038/s41440-018-0112-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0112-7
Keywords
This article is cited by
-
Visit-to-visit blood pressure variability and risk of adverse birth outcomes in pregnancies in East China
Hypertension Research (2021)