Abstract
The brain renin-angiotensin system plays a crucial role in ischemic stroke. It is known that stimulation of the angiotensin II type 2 (AT2) receptor protects against ischemic brain injury. We recently demonstrated that AT2 receptor stimulation by compound 21 (C21), a direct AT2 receptor agonist, inhibited vascular intimal proliferation with activation of peroxisome proliferator-activated receptor-gamma (PPAR-γ). However, whether direct AT2 receptor stimulation protects against ischemic brain injury via PPAR-γ activation is still unknown. 8-week-old male C57BL/6 J mice were subjected to middle cerebral artery (MCA) occlusion. 2 weeks before MCA occlusion, they were administered C21 with or without GW9662, a PPAR-γ antagonist. Neurologic deficit, ischemic size, superoxide anion, superoxide dismutase (SOD) activity, expression of NADPH subunits and blood brain barrier (BBB) stabilization were assessed 24 h after MCA occlusion. Cerebral blood flow (CBF) was measured in the core and periphery of the MCA territory before, immediately after, 1 h and 24 h after MCA occlusion. Treatment with C21 markedly decreased the neurologic deficit and ischemic size with an increase in CBF, SOD activity and BBB stabilization genes compared with the non-treated group. Co-administration of GW9662 partially attenuated this protective effect of C21 on neurologic deficit and ischemic size via an increase in superoxide anion production and a decrease of SOD activity and BBB stabilization genes, while GW9662 treatment alone had no significant effect on neurologic deficit and ischemic size. These results suggest that direct AT2 receptor stimulation has a preventive effect on stroke-induced brain injury partly due to activation of PPAR-γ.
Similar content being viewed by others
Introduction
Stroke is one of the leading causes of death and disability worldwide. It is known that the renin-angiotensin system (RAS) plays a crucial role in the pathophysiology of ischemic stroke [1, 2]. Angiotensin II binds with high affinity to two distinct receptors: angiotensin II type 1 (AT1) receptor and angiotensin II type 2 (AT2) receptor. The AT2 receptor is abundantly and widely expressed in fetal tissues, but its expression declines rapidly after birth [3, 4]. Interestingly, the AT2 receptor was shown to be up-regulated in the rat brain after transient ischemic stroke [5, 6]. AT2 receptor deficient mice exhibited larger infarct volume than wild-type mice [7], and beneficial actions of AT2 receptor modulation in the brain have been documented in experimental ischemic stroke [6, 8, 9].
A newly developed selective and potent non-peptide direct AT2 receptor agonist, compound 21 (C21) [10], has contributed to revealing neuroprotection through AT2 receptor stimulation, as confirmed using AT2 receptor-deficient mice [8] or an AT2 receptor blocker, PD 123319 [6], in experimental ischemic stroke. In the pre-clinical phase, C21 showed strong potential to be developed as treatment for stroke because of its oral availability and anticipated minimal effect on blood pressure.
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) is a nuclear transcription factor which plays a role in several diseases including obesity, diabetes, atherosclerosis and ischemic stroke [11]. Expression of PPAR-γ mRNA and protein were elevated after ischemic brain injury, and a PPAR-γ agonist demonstrated efficient neuroprotective in the rat brain after transient ischemic stroke [12,13,14,15]. PPAR-γ was proved to be an endogenous protective factor by the finding that treatment with a PPAR-γ antagonist increased infarct size [12]. Furthermore, a Cochrane Database study showed that PPAR-γ agonists appeared to prevent recurrent stroke and other vascular events in patients with stroke or transient ischemic attack [16].
Recently, we reported that direct AT2 receptor stimulation by C21 accompanied by PPAR-γ activation ameliorated insulin resistance in type 2 diabetic mice [17], and inhibited vascular intimal proliferation [18]. Our study and recent research have demonstrated that AT2 receptor-interacting protein (ATIP) was involved in AT2 receptor-induced PPAR-γ complex formation [18], and the crosstalk between AT2 receptor and PPAR-γ may be via Wnt10b/β-catenin signaling [19, 20]. Moreover, we demonstrated that an AT1 receptor antagonist exerted a protective effect against ischemic brain damage through PPAR-γ activation [21]. Studies from our laboratory and other groups have shown that AT1 receptor blockade mediated its beneficial effect on ischemic stroke through relative AT2 receptor stimulation [7, 22]. Taking these findings together, it is unconfirmed but suspected that direct AT2 receptor stimulation attenuates ischemic stroke damage via PPAR-γ activation.
Understanding the function of direct AT2 receptor stimulation under the pathological condition of ischemic stroke is crucial to reap its benefit. In our study, we aimed to investigate the preventive effect of pretreatment with C21 on acute neurologic injury in an ischemic stroke model, and elucidate the possible molecular mechanisms involving PPAR-γ.
Materials and methods
This study was performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. All animal studies were reviewed and approved by the Animal Studies Committee of Ehime University.
Animals and treatment
Adult male C57BL6/J mice (Clea Japan, Inc., Tokyo, Japan) were used in this study. Mice were housed in a room in which lighting was controlled (12 h on and 12 h off) and temperature was kept at 25 °C. They were given a standard diet (MF, Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. 8-week-old mice were treated with C21 (Vicore Pharma, Gothenburg, Sweden) intraperitoneally at 10 μg/kg/day with or without GW9662 (M6191, Sigma-Aldrich, St. Louis, MO) at 0.35 mg/kg/day in drinking water for 2 weeks according to previous reports [18, 23]. Control mice were only treated with saline intraperitoneally at 4 ml/kg/day for 2 weeks.
Systolic blood pressure measurement
Systolic blood pressure (SBP) was measured in conscious mice on the day before middle cerebral artery occlusion operation, by the tail-cuff method (MK-2000ST, Muromachi Kikai Co., Ltd., Tokyo, Japan) as described in a previous report [24]. Mice were held in a small plastic holder on a warm pad. Mean systolic blood pressure of ten measurements in each mouse was determined.
Middle cerebral artery occlusion model
Focal cerebral ischemia was induced by middle cerebral artery (MCA) occlusion using an intraluminal filament according to a method previously described [25]. Briefly, mice were anesthetized intraperitoneally with 84.52 mg/kg somnopentyl in saline. After making a midline neck incision, the left common and external carotid arteries were isolated and ligated. A nylon monofilament (Ethilon W1765, ETHICON, LLC., San Lorenzo, Puerto Rico, USA) coated with silicone resin (Provil novo, Heraeus Kulzer GmbH, Grüner Weg, Hanau, Germany) was inserted through a small incision in the common carotid artery and advanced to a position 9 mm distal to the carotid bifurcation, for occlusion of the MCA.
Ischemic area measurement
To measure the ischemic area after focal cerebral ischemia, the mouse brain was extracted 24 h after MCA occlusion, and sliced into seven coronal sections with 1-mm thickness and immediately stained with 2% 2,3,5-triphenyltetrasodium chloride (TTC; 35317–32, Nacalai Tesque, Inc., Kyoto, Japan) as previously described [26]. Ischemic area was defined as the TTC-unstained area. Results were presented as the ischemic ratio, the percentage of ischemic area to total brain area and the percentage of infarct volume to total brain volume.
Neurologic deficit evaluation
Neurologic deficit was evaluated 24 h after MCA occlusion using the neurologic score of Longa method as described previously [27]. Neurologic findings were scored on a five-point scale: a score of 0 indicates no neurologic deficit, 1 (failure to extend left forepaw fully) indicates a mild focal neurologic deficit, 2 (circling to the right) indicates a moderate focal neurologic deficit, 3 (falling to the right) indicates a severe focal deficit, and mice with a score of 4 did not walk spontaneously and had a depressed level of consciousness. Two observers were blinded to group assignment, and they performed the above protocol independently. Mice in each group were randomly assigned to observers to minimize variability in application of the scoring system between groups.
Cerebral blood flow monitoring
Cerebral blood flow (CBF) was measured with a two-dimensional laser speckle blood flow imager (Omegazone, Omegawave, Inc., Tokyo, Japan) as described previously [28]. Briefly, mice were anesthetized by intraperitoneal injection of 84.52 mg/kg somnopentyl in saline. A midline incision was made in the scalp to expose the skull. A 780 nm laser semiconductor was used to illuminate the area of interest. CBF in the core and periphery of the MCA territory was monitored before, immediately after, 1 and 24 h after MCA occlusion.
Real-time quantitative reverse-transcription polymerase chain reaction
Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR) was performed with a SYBR green I kit (MJ Research, Inc., Waltham, MA). mRNA was prepared from the cortex of the ipsilateral side 24 h after MCA occlusion for all the genes. The RT-PCR primers for genes were as follows: p22phox; 5′-TGGCTACTGCTGGACGTTTCAC-3′ (forward) and 5‵-CTCCAGGAGACAGATGAGCACAC-3‵ (reverse), p40phox; 5′-TTTGAGCAGCTTCCAGACGA-3′ (forward) and 5‵-GGTGAAAGGGCTGTTCTTGC-3‵ (reverse), p47phox; 5′-GTCCCTGCATCCTATCTGGA-3′ (forward) and 5‵-GGGACATCTCGTCCTCTTCA-3‵ (reverse), p67phox; 5′-CAGACCCAAAACCCCAGAAA-3′ (forward) and 5‵-AGGGTGAATCCGAAGCTCAA-3‵ (reverse), gp91phox; 5′-TGGGATCACAGGAATTGTCA-3′ (forward) and 5‵-CTTCCAAACTCTCCGCAGTC-3‵ (reverse), superoxide dismutase-1 (SOD-1); 5′-GAGACCTGGGCAATGTGACT-3′ (forward) and 5‵-GTTTACTGCGCAATCCCAAT-3‵ (reverse), SOD-2; 5′-CCGAGGAGAAGTACCACGAG-3′ (forward) and 5‵-GCTTGATAGCCTCCAGCAAC-3‵ (reverse), SOD-3; 5′-ATCCCACAAGCCCCTAGTCT-3′ (forward) and 5‵-GTGCTATGGGGACAGGAAGA-3‵ (reverse), AT1 receptor; 5′-AGTCGCACTCAAGCCTGTCT-3′ (forward) and 5‵-ACTGGTCCTTTGGTCGTGAG-3‵ (reverse), AT2 receptor; 5′-CACTGGCAACTAAAAAGGTGTAAG-3′ (forward) and 5‵-CGGCTGCTGGTAATGTTTCTG-3‵ (reverse), occludin; 5′-ACTGGGTCAGGGAATATCCA-3′ (forward) and 5‵-TCAGCAGCAGCCATGTACTC-3‵ (reverse), claudin-5; 5′-GGCGATTACGACAAGAAGAACT-3′ (forward) and 5‵-TAGTGATGGTCAACGGACTCTG-3‵ (reverse), zonula occludens (ZO)-1; 5′-ACTCCCACTTCCCCAAAAAC-3′ (forward) and 5‵-CCACAGCTGAAGGACTCACA-3‵ (reverse), endothelial nitric oxide synthase (eNOS); 5′-GGCTCCCTCCTTCCGGCTG-3′ (forward) and 5‵-TCCCGCAGCACGCCGAT-3‵ (reverse), vascular endothelial growth factor (VEGF); 5′-CACGACAGAAGGAGAGCAGAAGT-3′ (forward) and 5‵-TTCGCTGGTAGACATCCATGAA-3‵ (reverse), glyceraldehyde-3-phosphate dehydrogenase (GAPDH); 5′-TGCGACTTCAACAGCAACTC-3′ (forward) and 5‵-ATGTAGGCCATGAGGTCCAC- 3‵ (reverse).
Superoxide anion detection
Histologic detection of superoxide anion in the boundary zone of the infarcted cortical area was carried out as previously described [29]. In brief, frozen, enzymatically intact, 10-μm-thick sections were prepared from mouse brain 24 h after MCA occlusion, and incubated immediately with 5 μmol/L dihydroethidium (DHE; D23107, Invitrogen, Eugene, OR) for 15 min at 37 °C in a humidified chamber protected from light. DHE is oxidized by superoxide anion to ethidium, which binds to DNA in the nucleus and fluoresces red. For detection of ethidium, sections were observed with an Axioskop microscope (Axioskop 2 Plus with AxioCam, Carl Zeiss, Oberkochen, Germany) equipped with a computer-based imaging system. Fluorescence of ethidium was detected with a 590 nm long-pass filter. The intensity of the fluorescence was analyzed and quantified using computer-imaging software (Densitograph, ATTO Corp., Tokyo, Japan).
Superoxide dismutase activity assay
Total SOD activity was assayed with a SOD assay kit-WST (S311, Dojindo Laboratories, Kumamoto, Japan) 24 h after MCA occlusion according to the technical manual. In brief, the ipsilateral and contralateral ischemic cortex were each homogenized in 500 mL ice-cold extraction buffer (0.25 mol/L sucrose (196–00015, Wako, Osaka, Japan), 10 mmol/L HEPES (342–01375, Dojindo), 1 mmol/L EDTA (345–01865, Dojindo), pH 7.4). After samples were centrifuged (10,000 g, 60 min, 4 °C), 20 μL of the supernatant was incubated with assay reagent containing a water-soluble tetrazolium salt, WST-1, for 20 min at 37 °C. Superoxide anion reduced WST-1 to WST-1 diformazan, which absorbed maximally at 450 nm. SOD in samples inhibited the WST-1 reduction as it catalyzed the dismutation of superoxide anion. SOD activity was calculated as the amount of enzyme in the sample solution that inhibited the reduction reaction of WST-1 by 50%. Results were presented as specific activity, which was determined as the ratio of ipsilateral to contralateral activity. Data were standardized by the wet weight of cortex samples.
Statistical analysis
All values were presented as mean ± standard error of the mean in the text and figures. Data were evaluated by analysis of variance followed by post hoc analysis for multiple comparisons. A difference with P < 0.05 was considered significant.
Results
Effect of C21 to attenuate ischemic brain damage and improve neurological outcome partly via PPAR-γ activation after MCA occlusion
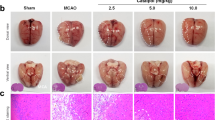
Body weight and SBP were not significantly affected by C21 and/or GW9662 treatment (Table 1). Brain sections were prepared 24 h after MCA occlusion, and ischemic area was determined by TTC staining (Fig. 1a). As shown in the histogram of ischemic ratio in each section of brain (Fig. 1b), in control mice the maximal ischemic area was about 25% of the total area around section 3, which is the main territory of the MCA, as previously described [7]. Regarding the average ischemic ratio, C21 treatment for 2 weeks significantly decreased the infarct volume (Fig. 1c). Co-administration of C21 with GW9662 attenuated the C21-induced decrease in infarct volume. However, treatment with GW9662 alone did not affect the infarct volume compared with the control group. Similar effects on the neurologic deficit 24 h after ischemic stroke were observed (Fig. 1d), Although treatment with C21 had a tendency to reduce mortality rate, there was no significant difference in the mortality rate among each group (data were not shown). C21 markedly improved the neurological outcome after ischemic stroke, while co-administration with GW9662 reversed it. In Fig. 1, GW9662 canceled the decrease of infarct volume and neurologic deficit by C21 totally, not in part. We have performed additional dose course of GW9662 to explore the dose which canceled the preventive effect of C21 in part. We found that 0.035 mg/kg/day (10-folds dilution from primary dose) was the partial inhibition dose for C21 (data were not shown).
Ischemic area and neurologic deficit 24 h after middle cerebral artery (MCA) occlusion. Mouse brains were taken 24 h after MCA occlusion. Coronal sections were stained with 2,3,5-triphenyltetrazolium chloride (TTC) (a). Ischemic area (b) was expressed as a percentage of total area and and infarct volume (c) was expressed as a percentage of total volume. Neurologic score was used to evaluate the neurologic deficit 24 h after MCA occlusion (d). CON control, C21 compound 21, GW GW9662. *P < 0.05 vs. CON; **P < 0.01 vs. CON; †P < 0.05 vs. C21; ††P < 0.01 vs. C21. n = 10–13 for each group for ischemia. n = 12–20 for each group for neurologic deficit. Values are mean ± SEM
Effect of C21 to increase CBF partly via PPAR-γ activation after MCA occlusion
CBF in the core and periphery of the MCA territory was measured before, immediately after, 1 and 24 h after MCA occlusion using a laser speckle blood flow imager. CBF decreased immediately after MCA occlusion, and this reduction continued for at least 24 h. C21 pretreatment with or without GW9662 had no significant effect on baseline CBF (CBF before MCA occlusion) (data were not shown). Time-course analysis of CBF showed that treatment with C21 significantly ameliorated CBF 24 h after MCA occlusion both in the core and peripheral region compared with the control group (Fig. 2a, b). The beneficial effect of C21 on CBF was markedly attenuated by co-treatment with GW9662, while CBF was not significantly changed in mice with GW9662 treatment alone.
Changes of cerebral blood flow in core and peripheral region of middle cerebral artery (MCA) territory before and after MCA occlusion. Cerebral blood flow (CBF) was determined immediately, 1, and 24 h after MCA occlusion by laser-Doppler flowmetry (a). Change of CBF was expressed as a percentage of basal flow (b). CON control, C21 compound 21, GW GW9662. *P < 0.05 vs. CON; **P < 0.01 vs. CON; †P < 0.05 vs. C21; ††P < 0.01 vs. C21. n = 9–14 for each group. Values are mean ± SEM
Effect of C21 to reduce superoxide anion production partly via PPAR-γ activation after MCA occlusion
To assess the involvement of oxidative stress in the exacerbation of focal brain ischemia, superoxide anion production in the area described in Fig. 3a was detected by DHE staining 24 h after MCA occlusion. Treatment with C21 markedly reduced superoxide anion production compared with the control group. However, co-administration of C21 with GW9662 significantly prevented this reduction (Fig. 3b). Treatment with GW9662 alone did not show a significant effect on superoxide anion production compared with the control group.
Superoxide anion production, superoxide dismutase (SOD) activity and SOD mRNA expression 24 h after middle cerebral artery (MCA) occlusion. Superoxide anion production was analyzed as the intensity of dihydroethidium (DHE) staining in fresh-frozen sections from the ischemic cortex (a, b). SOD activity determined as the ratio of the ipsilateral to the contralateral side (c). Expression of SOD mRNA (d–f). CON control, C21 compound 21, GW GW9662. *P < 0.05 vs. CON; **P < 0.01 vs. CON; †P < 0.05 vs. C21; #P < 0.05 vs. SHAM; ##P < 0.01 vs. SHAM. n = 7 for each group for superoxide anion production. n = 8 for each group for SOD activity. n = 3–7 for each group for SOD mRNA expression. Values are mean ± SEM
Effect of C21 to elevate SOD activity partly via PPAR-γ activation after MCA occlusion
To clarify the mechanism of the change in oxidative stress after C21 and/or GW9662 treatment, we examined the possibility that C21 may elevate SOD activity partly via PPAR-γ activation. The increased ratio of SOD activity on the ipsilateral to contralateral side 24 h after MCA occlusion is shown in Fig. 3c. SOD activity was significantly elevated by C21 treatment. This increase was markedly suppressed by addition of GW9662. However, we did not observe a significant effect of GW9662 alone on SOD activity.
Effect of C21 on expression of oxidative stress genes after MCA occlusion
A prominent difference in expression of oxidative stress genes, such as SOD and NADPH subunits, was not observed among each group either. C21 and/or GW9662 administration did not markedly affect AT1R and AT2R expression (Figs. 3d-f and 4).
Effect of C21 on cerebrovascular injuries after MCA occlusion
In order to figure out how C21 affected the CBF, we investigated the effect of C21 and/or GW9662 on cerebrovascular-related genes such as blood brain barrier (BBB) stabilization and endothelial cell protection. Treatment with C21 significantly increased the mRNA expression of occludin, claudin-5 and ZO-1, compared with the control group. However, the beneficial effect of C21 on BBB stabilization was canceled by co-treatment with GW9662 (Fig. 5a-c). On the other hand, C21 and/or GW9662 administration did not markedly affect eNOS and VEGF expression (Fig. 5d-e).
Gene expressions of blood brain barrier stabilization and endothelial cell protection genes 24 h after middle cerebral artery (MCA) occlusion. CON control, C21 compound 21, GW GW9662. *P < 0.05 vs. CON; †P < 0.05 vs. C21; #P < 0.05 vs. SHAM; ##P < 0.01 vs. SHAM. n = 4–5 for each group. Values are mean ± SEM
Discussion
The present study demonstrated that direct AT2 receptor stimulation has a preventive effect on ischemic stroke-induced brain injury partly due to activation of PPAR-γ.
Recently, we reported that direct AT2 receptor stimulation by C21 accompanied by PPAR-γ activation ameliorated insulin resistance in type 2 diabetic mice [17], and inhibited vascular intimal proliferation [18], indicating that PPAR-γ is a downstream factor in the signaling pathway after direct AT2 receptor stimulation. However, in the pathological process of ischemic stroke, the signaling pathway is still unknown. In our present study, pretreatment with C21 effectively prevented ischemic damage by increasing CBF and decreasing oxidative stress after MCA occlusion, while co-administration of GW9662 significantly counteracted the preventive effects of C21, revealing that PPAR-γ is regulated by AT2 receptor stimulation in ischemic stroke. Regarding the effect of GW9662, administration of GW9662 increased ischemic size in C21 treated group but not in non-treated group. In other word, GW9662 itself did not have additive effect on cerebral ischemia. We consider this discrepancy is explained as follows. In this study, mice were pretreated with C21 with or without GW9662 2 weeks before MCA occlusion. C21 may activate PPAR-γ-related signaling and co-administration of GW9662 prevented such C21-induced “activated” PPAR-γ-related signaling. On the other hand, treatment with GW9662 alone prevented “endogenous” PPAR-γ-related signaling. This discrepancy could be induced by C21-pretreatment-incduced change of basal situation before MCA occlusion via “activated” PPAR-γ-related signaling such as decrease in reactive oxygen species (ROS) production ability and increase in BBB stabilization genes or collateral circulation etc.
In ischemic stroke, the ischemic region can be separated into the infarct core, in which oxygen supply is too low to sustain cell viability, and the ischemic penumbra [30]. The penumbra, the severely hypoxic but potentially salvageable region surrounding the ischemic core, is the main target for brain protective therapy [31, 32]. In keeping with the results of our previous study [8], pretreatment with C21 effectively ameliorated the decrease of CBF in the peripheral regions of the MCA territory after MCA occlusion. In addition, co-administration of GW9662 significantly counteracted the improving effect of C21 on CBF, indicating that PPAR-γ is involved in CBF regulation. Previously we reported that C21 administration significantly decreased BBB permeability in the ischemic stroke [8], and AT1 receptor blockade with PPAR-γ activation helps with protection against cognitive decline by preserving the integrity of the BBB [33]. Coinciding with these results, pretreatment with C21 showed vascular-protective effects in the penumbra region via increased occludin, claudin-5 and ZO-1 expression with PPAR-γ activation.
Inflammation is a critical step in the ischemic stroke, while post-ischemic neuro-inflammation is a long-term factor for ischemic injury [34, 35]. Following brain ischemia, inflammatory responses are initiated as a result of several agents, such as ROS formation [35,36,37]. ROS is a major group of oxidants in the process of oxidative stress, and it is generated in the early stage of ischemic stroke [38]. Therefore we focused on the initial preventive effect of C21 on ischemic injury by evaluating the oxidative stress at a 24 h endpoint. Oxidative stress is considered to be involved in various pathological processes, like hypertension, which is the most common chronic disease and a major cause of stroke [39]. We previously reported that brain ischemia was accompanied by elevated superoxide anion production, while AT2 receptor stimulation by C21 [8] and activation of PPAR-γ by AT1 receptor blockade [21] significantly decreased oxidative stress. Coinciding with these results, in the present study, pretreatment with C21 markedly reduced superoxide anion production compared with the control group. Further, co-administration with GW9662 prevented this reduction, revealing that C21 decreased oxidative stress via PPAR-γ activation. The mechanism of these results was that C21 up-regulated SOD activity via PPAR-γ activation, while the expression of SOD genes was not affected. Other researchers reported that activation of intracerebral PPAR-γ showed a protective effect against ischemic injury through up-regulation of expression of SOD mRNA and protein in transient MCA occlusion [40, 41]. Unlike their results, a significant change in SOD mRNA expression was not observed in our study. It is known that ischemia-reperfusion results in more severe oxidative stress than does permanent ischemia [42, 43]. Because the oxidative stress in their transient MCA occlusion model was more severe than that in our permanent MCA occlusion model, a prominent change in SOD gene expression was not observed in our study. Shimazu et al. demonstrated that a PPAR-γ agonist, pioglitazone, reduces infarct size in mice with transient but not permanent MCA occlusion, and CuZn-SOD acts as a mediator of neuroprotection because an increase in CnZn-SOD is observed after pioglitazone treatment [40]. They also suggested that the role of PPAR-γ is specific to events occurring during reperfusion. The beneficial mechanism of PPAR-γ on ischemic brain damage is compatible with the results of our present study. If we used an ischemia-reperfusion model, more marked C21-induced brain protective effects may be obtained.
In our present study, MCA occlusion showed no significant effect on AT2 receptor expression, which was not consistent with the reports cited in the introduction [5, 6]. The most probable cause is that the former was transient MCA occlusion model, while it was permanent MCA occlusion model in our study. As above mentioned, ischemia-reperfusion resulted in different pathophysiology from permanent ischemia [42, 43].
Our findings suggest that pretreatment with C21 prevents brain damage after ischemic stroke partly due to PPAR-γ activation. This neural-protective effect of C21 may contribute to improving the quality of life in patients with ischemic stroke. Further clinical investigation is needed to confirm this beneficial role of C21.
References
Arroja MM, Reid E, McCabe C. Therapeutic potential of the renin angiotensin system in ischaemic stroke. Exp Transl Stroke Med. 2016;8:8.
Fouda AY, Artham S, El-Remessy AB, Fagan SC. Renin-angiotensin system as a potential therapeutic target in stroke and retinopathy: experimental and clinical evidence. Clin Sci. 2016;130:221–38.
Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc Natl Acad Sci USA. 1991;88:11440–4.
Nuyt AM, Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Ontogeny of angiotensin II type 2 receptor mRNA expression in fetal and neonatal rat brain. J Comp Neurol. 1999;407:193–206.
Kagiyama T, Kagiyama S, Phillips MI. Expression of angiotensin type 1 and 2 receptors in brain after transient middle cerebral artery occlusion in rats. Regul Pept. 2003;110:241–7.
Alhusban A, Fouda AY, Bindu P, Ishrat T, Soliman S, Fagan SC. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J Hypertens. 2015;33:170–80.
Iwai M, Liu HW, Chen R, et al. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110:843–8.
Min LJ, Mogi M, Tsukuda K, et al. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am J Hypertens. 2014;27:1036–44.
Schwengel K, Namsolleck P, Lucht K, et al. Angiotensin AT2-receptor stimulation improves survival and neurological outcome after experimental stroke in mice. J Mol Med. 2016;94:957–66.
Wan Y, Wallinder C, Plouffe B, et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008.
Derosa G, Sahebkar A, Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J Cell Physiol. 2018;233:153-61.
Victor NA, Wanderi EW, Gamboa J, et al. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–63.
Ou Z, Zhao X, Labiche LA, et al. Neuronal expression of peroxisome proliferator-activated receptor-gamma (PPARgamma) and 15d-prostaglandin J2--mediated protection of brain after experimental cerebral ischemia in rat. Brain Res. 2006;1096:196–203.
Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–75.
Lin TN, Cheung WM, Wu JS, et al. 15d-prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–7.
Liu J, Wang LN. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2015; CD010693.
Ohshima K, Mogi M, Jing F, et al. Direct angiotensin II type 2 receptor stimulation ameliorates insulin resistance in type 2 diabetes mice with PPARgamma activation. PLoS ONE. 2012;7:e48387.
Kukida M, Mogi M, Ohshima K, et al. Angiotensin II type 2 receptor inhibits vascular intimal proliferation with activation of PPARgamma. Am J Hypertens. 2016;29:727–36.
Matsushita K, Wu Y, Pratt RE, Dzau VJ. Deletion of angiotensin II type 2 receptor accelerates adipogenesis in murine mesenchymal stem cells via Wnt10b/beta-catenin signaling. Lab Invest. 2016;96:909–17.
Yuan Z, Li Q, Luo S, et al. PPARgamma and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2016;11:216–25.
Iwanami J, Mogi M, Tsukuda K, et al. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J Hypertens. 2010;28:1730–7.
Faure S, Bureau A, Oudart N, Javellaud J, Fournier A, Achard JM. Protective effect of candesartan in experimental ischemic stroke in the rat mediated by AT2 and AT4 receptors. J Hypertens. 2008;26:2008–15.
Tsukuda K, Mogi M, Iwanami J, et al. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009;54:782–7.
Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–5.
J-i Koizumi, Yoshida, Nakazawa Y, Ooneda T. G. Experimental studies of ischemic brain edema. 1. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8.
Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91.
Miller D, Forrester K, Leonard C, Salo P, Bray RC. ACL deficiency impairs the vasoconstrictive efficacy of neuropeptide Y and phenylephrine in articular tissues: a laser speckle perfusion imaging study. J Appl Physiol. 2005;98:329–33.
Szocs K, Lassegue B, Sorescu D, et al. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–7.
Hata R, Mies G, Wiessner C, et al. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–75.
Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11(Suppl 1):2–8.
Baron JC. Mapping the ischaemic penumbra with PET: a new approach. Brain. 2001;124(Pt 1):2–4.
Min LJ, Mogi M, Shudou M, et al. Peroxisome proliferator-activated receptor-gamma activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood-brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension. 2012;59:1079–88.
Nishi T, Maier CM, Hayashi T, Saito A, Chan PH. Superoxide dismutase 1 overexpression reduces MCP-1 and MIP-1 alpha expression after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:1312–24.
Kim JS, Gautam SC, Chopp M, et al. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995;56:127–34.
Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14.
Chen H, Kim GS, Okami N, Narasimhan P, Chan PH. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 2011;42:341–8.
Murakami K, Kondo T, Kawase M, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–13.
Solak Y, Afsar B, Vaziri ND, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. 2016;39:567–73.
Shimazu T, Inoue I, Araki N, et al. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–9.
Tureyen K, Kapadia R, Bowen KK, et al. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56.
Peters O, Back T, Lindauer U, et al. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1998;18:196–205.
Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–99.
Acknowledgements
This work was originally presented at the International Stroke Conference 2017, February 2017.
Funding
This study was supported by JSPS KAKENHI [Grant Number 25293310 to MH, 25462220 to MM, 15K19974 to JI, and 26860567 to LJM], and research grants from pharmaceutical companies: Astellas Pharma Inc., Bayer Yakuhin, Ltd., Daiichi-Sankyo Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K. K., Shionogi & Co., Ltd., and Takeda Pharmaceutical Co., Ltd. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shan, BS., Mogi, M., Iwanami, J. et al. Attenuation of stroke damage by angiotensin II type 2 receptor stimulation via peroxisome proliferator-activated receptor-gamma activation. Hypertens Res 41, 839–848 (2018). https://doi.org/10.1038/s41440-018-0082-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0082-9
This article is cited by
-
Direct AT2R Stimulation Slows Post-stroke Cognitive Decline in the 5XFAD Alzheimer’s Disease Mice
Molecular Neurobiology (2022)
-
Deterioration of cognitive function after transient cerebral ischemia with amyloid-β infusion—possible amelioration of cognitive function by AT2 receptor activation
Journal of Neuroinflammation (2020)
-
The Renin-Angiotensin System in the Central Nervous System and Its Role in Blood Pressure Regulation
Current Hypertension Reports (2020)
-
Delayed Administration of Angiotensin II Type 2 Receptor (AT2R) Agonist Compound 21 Prevents the Development of Post-stroke Cognitive Impairment in Diabetes Through the Modulation of Microglia Polarization
Translational Stroke Research (2020)
-
The Brain AT2R—a Potential Target for Therapy in Alzheimer’s Disease and Vascular Cognitive Impairment: a Comprehensive Review of Clinical and Experimental Therapeutics
Molecular Neurobiology (2020)