Abstract
Hypertension has strong adverse effects on cardiovascular diseases, and increased blood pressure (BP) variability (BPV) is closely associated with the development of hypertensive organ injuries and the occurrence of cardiovascular diseases. Similar to other forms of BPV, short-term beat-to-beat BPV has also been established as a risk factor for cardiovascular diseases. Baroreflex failure is the major mechanism involved in the pathophysiology of short-term beat-to-beat BPV. Previous clinical and animal studies have demonstrated that baroreflex failure disrupted beat-to-beat BPV and hypertensive organ damage. Moreover, short-term beat-to-beat BPV was an independent determinant of vascular elasticity. Although, the clinical measurement tools and therapeutics for beat-to-beat BPV are not sufficient, we should consider that large beat-to-beat BPV is an important risk factor for cardiovascular diseases in patients with hypertension.
Similar content being viewed by others
Introduction
Hypertension has strong adverse effects on cardiovascular diseases. Moreover, increased blood pressure (BP) variation (BPV) is closely associated with the development of hypertensive organ injuries and the occurrence of cardiovascular diseases [1,2,3]. There are several types of BPV, which are classified according to the cycle length [4]. The longest BPV is the seasonal variation. BP is generally highest in the winter and lowest in the summer. The BPV with most abundant information is diurnal changes evaluated by 24-h ambulatory monitoring or home BP. Masked hypertension, including morning surge and non-dipper, cause cardiovascular organ injuries and events [4]. Interestingly, BPV is modified by various factors, such as aging, external stress, diet, cardiovascular disease, or hypertension. To assess BPV, the standard deviation (SD) and the coefficient of variation (CV) of the systolic, diastolic, or mean BP have been used traditionally [5].
The SD of the BP measurements is commonly used as the clinical evaluation of short-term BPV [1]. However, we should realize that SD has limitations in estimating BPV because SD only reflects the global fluctuation of the BP values around the mean level and does not take into account the order of BP measurements [6,7,8]. To overcome these limitations of SD, average real variability (ARV), residual standard deviation (RSD) and variation independent of the mean (VIM) have been used [5]. ARV takes into account the sequence of BP measurements [5, 6]. RSD indicates the underlying trend between BP values and time [5, 7, 8]. VIM can exclude the impact of mean BP levels [5, 7].
Regarding the pathophysiological mechanisms of short-term BPV, previous studies have suggested the importance of baroreflex control [5, 9,10,11]. Considering this background, I have reviewed the clinical aspects of short-term beat-to-beat BPV, especially the association with baroreflex.
Beat-to-beat BPV as a risk factor for hypertension
Elevated BP is an important risk factor for target-organ damage [12]. Among the types of BPV, short-term (24-h and beat-to-beat) BPV has been established as a risk factor for cardiovascular diseases [4, 13,14,15]. Previously, several studies have demonstrated that 24-h BPV is significantly associated with vascular damage [12, 15,16,17]. Although beat-to-beat BPV is considered more precise than 24-h BPV to evaluate short-term BPV [15, 18], the association between beat-to-beat BPV and vascular damage is not significant [15]. Short-term beat-to-beat BPV evaluated by tonometry has also demonstrated a significant relationship with left ventricular mass index, urinary albumin excretion, cerebral white matter lesions, and carotid artery intima-media thickness in patients with hypertension [15, 19,20,21]. In addition, increased beat-to-beat BPV is associated with cardiovascular death in patients with stroke [22].
It is necessary to consider that BPV exhibits a close interaction with intrinsic cardiovascular-regulatory mechanisms and external environmental stimuli. Beat-to-beat BPV is mainly mediated by sympathetic nerve activity, arterial, or cardiopulmonary reflex, humoral factors, behavior, emotional factors, activity, and sleep [4]. The severity of hypertensive organ damage was augmented with increased mean BP and beat-to-beat BPV in 108 untreated hypertensive subjects [18]. The urinary albumin-to-creatinine ratio was significantly associated with beat-to-beat diastolic BPV [19]. However, systolic and diastolic BPV were not correlated with left ventricular mass index in 33 untreated hypertensive subjects [19], and a weak positive correlation was noted between short-term BPV and left ventricular mass index [23]. Left ventricular mass index is used to evaluate cardiac organ damage, and the urinary albumin-to-creatinine ratio was also used as a surrogate marker for renal organ damage. In the above controversial studies, Parati et al. [18] only used SD of BP values to evaluate the BPV, and the clinical study of Veerman et al. [19] included a small number of patients. Furthermore, in 256 untreated hypertensive subjects, Wei et al. [15] demonstrated that left ventricular mass index increased with the three indices of systolic BPV, and the urinary albumin-to-creatinine ratio only increased with differences between maximum and minimum systolic BP. None of the three indices of systolic BPV was significantly associated with pulse wave velocity. It has been demonstrated that all four indices of systolic BPV were associated with vascular elasticity and that SD, RSD, and VIM of diastolic BP were also correlated with total arterial compliance. In multivariate linear regression analysis, only VIM of systolic BP was associated with total arterial compliance independent of systolic and diastolic BP, age, and body mass index in a hypertensive population. However, BPV was controversially used to predict the risk of cardiovascular diseases [12, 15, 18, 19, 24,25,26]. When the relationship between BP and time was approximately linear, RSD was more suitable than SD to estimate the extent of variability. VIM is a transformation of SD that was defined to be uncorrelated with mean levels for all individuals in the cohort [8], and VIM could eliminate the confounding interference of the mean BP values [5]. Only one prospective study addressed the incidence of cardiovascular complications in relation to BP levels as assessed by home invasive intra-arterial 24-h ambulatory recordings in patients with hypertension. However, this study did not present the assessment of BPV [27, 28]. Based on the findings from the Northwick Park Heart Study [15, 29], one might hypothesize that the ability of beat-to-beat recordings to demonstrate associations with target organ damage might depend on the recording technique (intra-arterial vs. finger plethysmography) and the duration of the recordings (10 min vs. 24 h) [5].
In an animal study, rats with sinoaortic denervation were used as the established model of increased beat-to-beat BPV [30,31,32]. Sinoaortic denervation augments beat-to-beat BPV with the impairment of baroreflex sensitivity in the rats. It has been reported that sinoaortic denervation facilitates the development of hypertensive organ injuries in the heart, kidney, and small arteries of hypertensive rats without affecting the 24-h mean BP level [31]. In normotensive rats, sinoaortic denervation impaired left ventricular diastolic function and caused left ventricular wall thickening with cardiac fibrosis. We also demonstrated that sinoaortic denervation destabilized BP in conscious rats with normal left ventricular function and that salt loading combined with the loss-of-baroreflex increases the BPV irrespective of left ventricular function [32]. Regarding the mechanism of the effects of sinoaortic denervation on the cardiovascular system, tissue concentrations of angiotensin II increased in the heart and the kidney of rats after long-term sinoaortic denervation; however, plasma concentrations of angiotensin II were not altered [30]. It has been also reported that the mineral corticoid receptor is activated in arterial medial cells and cardiomyocytes in rats with sinoaortic denervation [33]. These results potentially suggested that short-term beat-to-beat BPV enhances the renin-angiotensin system in cardiovascular tissues. Regarding therapeutics, selective β1 blockers inhibit renin secretion and attenuated short-term BPV with the alleviation of cardiovascular tissue and organ injuries in rats with sinoaortic denervation [34].
Another pathophysiological mechanism associated with beat-to-beat BPV is vascular elastcity [35]. Abnormal vascular elasticity indicates alterations in the structural and functional vascular properties, and beat-to-beat BPV is exacerbated by structural and functional vascular properties in a hypertensive population.
Baroreflex control of blood pressure
Baroreflex is the fastest negative feedback system to stabilize BP [10, 11, 36,37,38]. Arterial baroreceptors are stretch receptors located within the arterial wall of elastic vessels, such as the aortic arch and carotid sinuses. Baroreflex senses BP, and activated afferent nerves relay the pressure signal to the vasomotor center. The vasomotor center changes the mechanical properties of the ventricle and vascular system to stabilize BP through modulating the autonomic nervous system [39,40,41,42,43,44]. Moreover, we demonstrated that baroreflex failure markedly impairs the volume load tolerance and predisposes individuals to pulmonary edema in rats without left ventricular dysfunction [45]. Arterial baroreflex failure is associated with heart failure with preserved but reduced left ventricular ejection fraction [46], and left ventricular diastolic dysfunction is induced by arterial baroreflex failure [47]. Numerous studies have revealed that major risk factors of heart failure with preserved left ventricular ejection fraction, such as aging, hypertension, diabetes, renal insufficiency, and atherosclerosis, are closely associated with arterial baroreflex failure [48,49,50,51,52,53]. Interestingly, baroreflex failure caused by prenatal hypoxia might be partially associated with the development of hypertension in adulthood [54].
Baroreflex and short-term beat-to-beat BPV
We sought to assess whether arterial baroreflex failure alone could strikingly disrupt BPV in the context of normal cardiac function [32, 45]. We isolated the vasculature of bilateral carotid sinuses from the systemic circulation and controlled carotid sinus pressure using a servo-controlled piston pump. Bilateral vagal nerves were sectioned at the middle of the neck to eliminate reflexes from the cardiopulmonary region and prevent efferent conduction. Bilateral aortic depressor nerves were sectioned to eliminate reflexes from the aortic arch. Using this preparation, we mimicked normal arterial baroreflex by matching carotid sinus pressure to instantaneous BP and arterial baroreflex failure by maintaining carotid sinus pressure at a constant value regardless of BP perturbations. In the context of normal arterial baroreflex function, volume loading increased BP, but the changes were relatively small. However, in the mimicked conditions of arterial baroreflex failure, the considerably smaller volume loading increased BP markedly.
As described above, we demonstrated that sinoaortic denervation destabilized BP in conscious rats with normal left ventricular function and that salt loading combined with loss-of-baroreflex increases BPV irrespective of left ventricular function [32]. Recently, we demonstrated that baroreflex-induced dynamic BP changes can be accurately predicted by the transfer function from carotid sinus pressure to mechanical properties and that changes in vascular properties, not the ventricular properties, predominantly determine baroreflex-induced BP regulation [55]. Major variables that contribute to BP changes include vascular properties, such as arterial resistance and stressed blood volume. In contrast, the impact of the changes in ventricular properties on BP regulation is remarkably small. We concluded that the vascular system, not the ventricular system, is the dominant actuator in baroreflex regulation of BP at least in the setting of normal cardiac function [55]. We reported that incorporating an artificial bionic baroreflex system in rats with baroreflex failure restored the disrupted BPV to a similar extent as the native baroreflex in acute experiments [45].
Therapies for beat-to-beat BPV
In the clinic, angiotensin-converting enzyme inhibitors improved baroreflex sensitivity and reduced short-term BPV [56]. In animal study, beta-blockers, which inhibit renin secretion via the beta1 adrenoreceptor, attenuate short-term BPV and are expected to alleviate cardiovascular tissue and organ injuries in the context of sinoaortic denervation [34]. Aliskiren and l-arginine treatments restored depressed baroreflex sensitivity in renovascular hypertensive rats [57]. Interestingly, a sodium-glucose co-transporter 2 inhibitor at a non-depressor dose ameliorated BP lability associated with sympathoinhibition during the active period and improved baroreflex sensitivity in streptozotocin-induced diabetes mellitus rats [58]. Regarding non-pharmacological treatments, vagal afferent nerve activation improved baroreflex function and is expected to reverse the impaired beat-to-beat BPV [59].
Baroreflex activation therapy
Baroreflex activation therapy is a novel technique for treating patients with resistant hypertension. The DEBuT-HT study (Device-Based Therapy of Hypertension) demonstrated a substantial and sustained reduction in blood pressure over a 3-month period in treatment-resistant hypertensive patients [60]. Subsequently, the Rheos Pivotal Trial evaluated the effect of baroreflex activation therapy in a double-blind, randomized, prospective, sham-controlled trial in which patients were randomized to receive baroreflex activation therapy either immediately or 6 months after implantation of the Rheos device [61]. A second-generation Rheos implantable baroreflex activation therapy device also reduced office BP at 6 months in a single-arm, open-label study (Barostim neo trial) [62]. Baroreflex activation therapy recently demonstrated a sustained effect on blood pressure after 6 years of follow-up [63].
Our novel bionic baroreceptor senses BP and translates BP into neuro-stimulation [64]. The physiological volume intolerance described above was fully reversed in a model with baroreflex failure [10, 45]. Our bionic arterial system fully reversed short-term BPV and the physiologic volume intolerance in mimicked arterial baroreflex failure. The potential for this artificial bionic baroreflex system to be used as a novel therapeutic tool for disrupted BPV and heart failure should be considered in the near future [45].
Conclusions
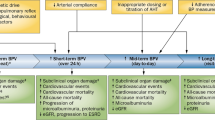
Short-term beat-to-beat BPV is one of the important clinical features of hypertension and is associated with baroreflex failure and vascular stiffening. Although, the clinical measurement tools and therapeutics for beat-to-beat BPV are insufficient, we should consider that large beat-to-beat BPV is a risk for cardiovascular diseases (Fig. 1).
References
Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyriw P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension findings from 2 large databases. Hypertension. 2012;60:369–77.
Gijón-Conde T, Graciani A, López-García E, Guallar-Castillón P, García-Esquinas E, Rodríguez-Artalejo F, Banegas JR. Short-term variability and nocturnal decline in ambulatory blood pressure in normotension, white-coat hypertension, masked hypertension and sustained hypertension: a population-based study of older individuals in Spain. Hypertens Res. 2017;40:613–9.
Isobe S, Ohashi N, Ishigaki S, Tsuji N, Tsuji T, Kato A, Yasuda H. Increased nocturnal blood pressure variability is associated with renal arteriolar hyalinosis in normotensive patients with IgA nephropathy. Hypertens Res. 2017;40:921–6.
Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood pressure variability. Nat Rev Cardiol. 2013;10:143–55.
Asayama K, Wei FF, Liu YP, Hara A, Gu YM, Shutte R, Li Y, Thijs L, Staessen JA. Does blood pressure variability contribute to risk stratification? Methodological issues and a review of outcome studies based on home blood pressure. Hypertens Res. 2015;38:97–101.
Mena L, Pintons S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–11.
Rothwell PM, Howard SC, Dolan W, O’Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS, ASCOT-BPLA and MRC Trial Investigators. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–80.
Rothwell PM, Howard SC, Dolan W, O’Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905.
Ishimitsu T. Beat-to-beat blood pressure variation and cardiovascular organ injuries in hypertension. Circ J. 2014;78:2162–3.
Kishi T. Regulation of sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 Academic Conference Award from the Japanese Society of Hypertension. Hypertens Res. 2013;36:845–51.
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46.
Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schilliaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2017;50:325–32.
Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow up study. Circulation. 2000;102:1536–41.
Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, Ferrario M, Mancia G. Blood pressure variability and organ damage in a general population: results from the PAMELA Study (Pressioni Arteriose Monitorate E Loro Associazioni). Hypertension. 2002;39:710–4.
Wei FF, Liu Y, Zhang L, Xu TY, Ding FH, Wang JG, Staessen JA. Beat-to-beat, reading-to-reading, and day-to-day blood pressure variability in relation to organ damage in untreated Chinese. Hypertension. 2014;63:790–6.
Stabouli S, Papakatsika S, Kotronis G, Papadopoulou-Legbelou K, Rizos Z, Kotsis V. Arterial stiffness and SBP variability in children and adolescents. J Hypertens. 2015;33:88–95.
Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costa B, Scherz R, Bond G, Zanchetti A. ELSA Investigators. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2001;19:1981–9.
Parati G, Pomidossi G, Albini FA, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–8.
Veerman DP, de Blok K, van Montfrans A. Relationship of steady state and ambulatory blood pressure variability to left ventricular mass and urinary albumin excretion in essential hypertension. Am J Hypertens. 1996;9:455–60.
Gómez-Angelats E, de La Sierra A, Sierra C, Parati G, Mancia G, Coca A. Blood pressure variability and silent cerebral damage in essential hypertension. Am J Hypertens. 2004;17:696–700.
Manios E, Michas F, Stamatelopoulos K, Barlas G, Koroboki E, Tsouma I, Vemmos K, Zakopoulos N. Short-term beat-to-beat but not ambulatory blood pressure variability is correlated to carotid intima-media thickness. Blood Press Monit. 2014;19:288–93.
Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke. 2000;31:463–8.
Madden JM, O’Flynn AM, Fitzgerald AP, Kearney PM. Correlation between short-term blood pressure variability and left-ventricular mass index: a meta-analysis. Hypertens Res. 2016;39:171–7.
Pierdomenico SD, Lapenna D, Di Tommaso R, Di Carlo S, Esposito AL, Di Mascio R, Ballone E, Cuccirullo F, Mezzetti A. Blood pressure variability and cardiovascular risk in treated hypertensive patients. Am J Hypertens. 2006;19:991–7.
Hansen TW, Thijs L,Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA, International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 h in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–57.
Schutte R, Thijs L, Liu YP, Asayama K, Jin Y, Odili A, Gu YM, Kuznetsova T, Jacobs L, Staessen JA. Within-subject blood pressure level–not variability–predicts fatal and nonfatal outcomes in a general population. Hypertension. 2012;60:1138–47.
Acharya DU, Heber ME, Dore CJ, Raftery EB. Ambulatory intraarterial blood pressure in essential hypertension. Effects of age, sex, race, and body mass—the Northwick Park Hospital Database Study. Am J Hypertens. 1996;9:943–52.
Khattar RS, Swales JD, Banfield A, Dore C, Senior R, Lahiri A. Prediction of coronary and cerebrovascular morbidity and mortality by direct continuous ambulatory blood pressure monitoring in essential hypertension. Circulation. 1999;100:1071–6.
Penaz J. Criteria for set point estimation in the volume clamp method of blood pressure measurement. Physiol Res. 1992;41:5–10.
Aoki Y, Kai H, Kajimoto H, Kudo H, Takayama N, Yasuoka S, Anegawa T, Iwamoto Y, Uchikawa H, Fukuda K, Kato S, Fukumoto Y, Imaizumi T. Large blood pressure variability aggravates arteriosclerosis and cortical sclerotic changes in the kidney in hypertensive rats. Circ J. 2014;78:2284–91.
Shan ZZ, Dai SM, Su DF. Arterial baroreflex deficit induced organ damage in sinoaortic denervated rats. J Cardiovasc Pharmacol. 2001;38:427–37.
Sakamoto K, Hosokawa K, Saku K, Sakamoto T, Tobushi T, Oga Y, Kishi T, Ide T, Sunagawa K. Baroreflex failure increases the risk of pulmonary edema in conscious rats with normal left ventricular function. Am J Physiol Heart Circ Physiol. 2016;310:H199–205.
Yasuoka S, Kai H, Kajimoto H, Kudo H, Takayama N, Anegawa T, Koga M, Miyamoto T, Mifune H, Kage M, Hirooka Y, Imaizumi T. Blood pressure variability activates cardiac mineralocorticoid receptor and induces cardiac remodeling in hypertensive rats. Circ J. 2013;77:1474–81.
Bertera FM, Del Mauro JS, Lovera V, Chiappetta D, Polizio AH, Taira CA, Hocht C. Acute effects of third generation β-blockers on shortterm and beat-to-beat blood pressure variability in sinoaortic-denervated rats. Hypertens Res. 2013;36:349–55.
Xia Y, Liu X, Wu D, Xiong H, Ren L, Xu L, Wu W, Zhang H. Influence of beat-to-beat blood pressure variability on vascular elasticity in hypertensive population. Sci Rep. 2017;7:8394.
Cowley AW Jr. Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300.
Ikeda Y, Kawada T, Sugimachi M, Kawaguchi O, Shishido T, Sato T, Miyano H, Matsuura W, Alexander J Jr, Sunagawa K. Neural arc of baroreflex optimizes dynamic pressure regulation in achieving both stability and quickness. Am J Physiol Heart Circ Physiol. 1996;271:H882–90.
Sato T, Kawada T, Inagaki M, Shishido T, Sugimachi M, Sunagawa K. Dynamics of sympathetic baroreflex control of arterial pressure in rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R262–70.
Kubota T, Alexander J Jr, Itaya R, Todaka K, Sugimachi M, Sunagawa K, Nose Y, Takeshita A. Dynamic effects of carotid sinus baroreflex on ventriculoarterial coupling studied in anesthetized dogs. Circ Res. 1992;70:1044–53.
Liu HK, Guild SJ, Ringwood JV, Barrett CJ, Leonard BL, Nguang SK, Navakatikyan MA, Malpas SC. Dynamic baroreflex control of blood pressure: influence of the heart vs. peripheral resistance. Am J Physiol Regul Integr Comp Physiol. 2002;283:R533–42.
Sarnoff SJ, Gilmore JP, Brockman SK, Mitchell JH, Linden RJ. Regulation of ventricular contraction by the carotid sinus. Its effect on atrial and ventricular dynamics. Circ Res. 1960;8:1123–36.
Shoukas AA, Brunner MC. Epinephrine and the carotid sinus baroreceptor reflex. Influence on capacitive and resistive properties of the total systemic vascular bed of the dog. Circ Res. 1980;47:249–57.
Suga H, Sagawa K, Kostiuk DP. Controls of ventricular contractility assessed by pressure-volume ration, Emax. Cardiovasc Res. 1976;10:582–92.
Vatner SF, Higgins CB, Franklin D, Braunwald E. Extent of carotid sinus regulation of the myocardial contractile state in conscious dogs. J Clin Invest. 1972;51:995–1008.
Funakoshi K, Hosokawa K, Kishi T, Ide T, Sunagawa K. Strikingly volume intolerance is induced by mimicking arterial baroreflex failure in normal left ventricular function. J Card Fail. 2014;20:53–9.
Banerjee P, Clark AL, Nikitin N, Cleland JG. Diastolic heart failure. Paroxysmal or chronic? Eur J Heart Fail. 2004;6:427–31.
Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–85.
Mostarda C, Moraes-Silva IC, Moreira ED, Medeiros A, Piratello AC, Comsolim-Colombo FM, Caldini EG, Brum PC, Krieger EM, Irigoyen MC. Baroreflex sensitivity impairment is associated with cardiac diastolic dysfunction in rats. J Card Fail. 2011;17:519–25.
Kaushal P, Taylor JA. Inter-relations among declines in arterial distensibility, baroreflex function and respiratory sinus arrhythmia. J Am Coll Cardiol. 2002;39:1524–30.
Protogerou AD, Stergiou GS, Lourida P, Achimastos A. Arterial stiffness and orthostatic blood pressure changes in untreated and treated hypertensive subjects. J Am Soc Hypertens. 2008;2:372–7.
Ueno LM, Miyachi M, Matsui T, Takahashi K, Yamazaki K, Hayashi K, Onodera S, Moritani T. Effect of aging on carotid artery stiffness and baroreflex sensitivity during head-out water immersion in man. Braz J Med Biol Res. 2005;38:629–37.
Kliger C, King DL, Maurer MS. A clinical algorithm to differentiate heart failure with a normal ejection fraction by pathophysiologic mechanism. Am J Geriat Cardiol. 2006;15:50–7.
Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med. 2004;55:373–94.
Svitok P, Molcan L, Stebelova K, Vesela A, Sedlackova N, Ujhazy E, Mach M, Zeman M. Prenatal hypoxia in rats increased blood pressure and sympathetic drive of the adult offspring. Hypertens Res. 2016;39:501–5.
Sakamoto T, Kakino T, Sakamoto K, Tobushi T, Tanaka A, Saku K, Hosokawa K, Onitsuka K, Murayama Y, Tsutsumi T, Ide T, Sunagawa K. Changes in vascular properties, not ventricular properties, predominantly contribute to baroreflex regulation of arterial pressure. Am J Physiol Heart Circ Physiol. 2015;308:H49–58.
Milovanovic B, Trifunovic D, Djuric D. Autonomic nervous system adjustment (ANSA) in patients with hypertension treated with enalapril. Acta Physiol Hung. 2011;98:71–84.
Mengal V, Silva PH, Tiradentes RV, Santuzzi CH, de Almeida SA, Sena GC, Bissoli NS, Abreu GR, Gouvea SA. Aliskiren and l-arginine treatments restore depressed baroreflex sensitivity and decrease oxidative stress in renovascular hypertension rats. Hypertens Res. 2016;39:769–76.
Yoshikawa T, Kishi T, Shinohara K, Takesue K, Shibata R, Sonoda N, Inoguchi T, Sunagawa K, Tsutsui H, Hirooka Y. Arterial pressure lability is improved by sodium-glucose co-transporter 2 inhibitor in streptozotocin-induced diabetic rats. Hypertens Res. 2017;40:646–51.
Saku K, Kishi T, Sakamoto K, Hosokawa K, Sakamoto T, Murayama Y, Kakino T, Ikeda M, Ide T, Sunagawa K. Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiol Rep. 2014;2:e12136.
Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Phillipp T, de Leeuw PW. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol. 2010;56:1254–8.
Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal trial. J Am Coll Cardiol. 2011;58:765–73.
Hoppe UC, Brandt MC, Wachter R, Beige J, Rump LC, Kroon AA, Cates AW, Lovert EG, Haller H. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens. 2012;6:270–6.
De Leeuw PW, Bisognano JD, Bakris GL, Nadim MK, Haller H,Kroon AA, on behalf of the DEBuT-HT and Rheos Trial Investigators. Sustained reduction of blood pressure with baroreceptor activation therapy: results of the 6-year open follow-up. Hypertension. 2017;69:836–43.
Hosokawa K, Ide T, Tobushi T, Sakamoto K, Onitsuka K, Sakamoto T, Fujino T, Saku K, Sunagawa K. Bionic baroreceptor corrects postural hypotension in rats with impaired baroreceptor. Circulation. 2012;126:1278–85.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to Kishi (C70423514) and partially by a Naito Foundation Research Grant. We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kishi, T. Baroreflex failure and beat-to-beat blood pressure variation. Hypertens Res 41, 547–552 (2018). https://doi.org/10.1038/s41440-018-0056-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0056-y
This article is cited by
-
Effects of vasodilators on beat-to-beat and every fifteen minutes blood pressure variability induced by noradrenaline infusion in rats
Hypertension Research (2024)
-
Short- to long-term blood pressure variability: Current evidence and new evaluations
Hypertension Research (2023)
-
The association between 24-h blood pressure variability and major adverse cardiac events (MACE) in hospitalized patients with acute myocardial infarction: a retrospective cohort study
The Egyptian Heart Journal (2021)
-
Blood pressure variability: its relevance for cardiovascular homeostasis and cardiovascular diseases
Hypertension Research (2020)
-
Blood pressure and heart rate variability and baroreflex sensitivity in white-coat, masked, and sustained hypertension
Hypertension Research (2020)