Abstract
The N-terminus of the PRNP gene normally contains a 5-octapeptide repeat (R1-R2-R2-R3-R4), and insertions at this locus can cause hereditary prion diseases. In the present study, we found a 5-octapeptide repeat insertion (5-OPRI) in a sibling case of frontotemporal dementia. Consistent with previous literature, 5-OPRI rarely met the diagnostic criteria for Creutzfeldt‒Jakob disease (CJD). We propose 5-OPRI as a suspected causative mutation for early-onset dementia, especially the frontotemporal type.
Similar content being viewed by others
Creutzfeldt‒Jakob disease (CJD) is a prion protein-mediated zoonosis that typically presents with subacute progressive dementia, leading to akinesia and mutism. Since abnormal prion proteins are more resistant to sterilization by heat and protein denaturation than viruses and bacteria, the prevention of prion infection is of the utmost importance. In Japan, prion disease surveillance began in 1999 to identify all patients with prion diseases, and prion-related protein analyses using cerebrospinal fluid (CSF) and PRNP gene analysis have continued as important diagnostic tests. The N-terminus of the PRNP gene normally contains an octapeptide repeat (R1-R2-R2-R3-R4). Insertions in this repeat have been reported to cause hereditary prion diseases in a small number of cases1. This mutation is rarer than the causative mutations for some other hereditary prion diseases, and its clinical traits depend on the number of repeat insertions; however, the details remain unclear, especially in cases with 5-octapeptide repeat insertion (5-OPRI)2. Recently, we found a case of siblings with 5-OPRI in which diagnosis was possible only by genetic analysis. We propose 5-OPRI as a suspected causative genetic mutation for early-onset dementia, especially the behavioral variant of frontotemporal dementia.
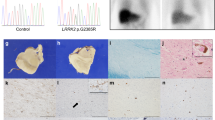
Patient 1 (Fig. 1a: III–1) had been aware of memory loss and calculation difficulties since the age of 56. His work colleagues mentioned that his behavior was impulsive, compulsive, and socially inappropriate (e.g., deviating from typical manners). He was diagnosed with Parkinsonism at the age of 58 years by a family physician and visited a neurology hospital. The patient’s father (Patient 3, Fig. 1a: II–1) was diagnosed with Pick’s disease, and the paternal grandmother (Fig. 1: I–2) was diagnosed with dementia at the age of 50 years and died at 61. Patient 1 showed conspicuously strange behavior and cognitive decline (Mini-Mental State Examination (MMSE) 22/30, Frontal Assessment Battery (FAB) 13/18, Raven’s Colored Progressive Matrices (RCPM) 25/37), a positive Myerson sign, cogwheel muscle rigidity in both upper limbs, and an unstable gait. There was no myoclonus detected. Blood tests revealed no abnormalities, and CSF tests revealed no abnormalities in cell count, total protein, total tau protein, 14-3-3 protein, or real-time quaking-induced conversion (RT-QUIC). Electroencephalography (EEG) revealed an 8–11 Hz fluctuation in the basic rhythm but no paroxysm, including periodic synchronous discharge (PSD). Diffusion-weighted imaging (DWI) revealed mild cortical atrophy in the frontotemporal lobe and enlargement of the lateral and third ventricles without cortical hyperintensity (Fig. 1b, c).
Patient 2 (Fig. 1: III–2) was the younger brother of patient 1. He had had frequent falls since the age of 41 years, and his family doctor diagnosed dementia with disorientation at the age of 42. He showed parkinsonian symptoms at the age of 47 years, and from the age of 48, he developed delirium and agitation at night, as well as socially inappropriate and compulsive behavior, behavioral inertia, and diminishing social interest. At 51 years of age, he could not walk, and at 54, he became bedridden. He was admitted to the neurology hospital at the age of 54 years for a thorough examination. He had difficulty understanding instructions due to severe cognitive impairment and showed a positive Myerson sign and palmar-mandibular reflex, tonicity in both upper limbs, hyperreflexia of tendons in both lower limbs, and positive abnormal reflexes. Blood test results were normal, and CSF examination revealed a normal cell count, elevated total protein (90 mg/dl), normal total tau protein, 14-3-3 protein, and a negative finding on RT-QUIC. EEG showed a basic rhythm of 8–11 Hz without paroxysms, including PSD. DWI revealed generalized atrophy of the cerebral cortex and ventricular enlargement without cortical hyperintensity (Fig. 1d, e).
Patient 3 was the father of patients 1 and 2. He presented with impulsive behavior, personality changes that had diminished his personal warmth, frequent falls, and repetitive movements at the age of 49; years, dysarthria at age 52; and gait disturbance and dementia at age 53. At the initial visit to a neurologist at the age of 56 years, he had severe dementia, frontal release signs including palmomental reflex and grasping, repetitive movements, dysarthria, dressing apraxia, hyperreflexia of both upper limb tendons, and impairment of the postural reflexes. EEG revealed a basic rhythm of 9–11 Hz with no paroxysms, including PSD. Brain computed tomography revealed generalized atrophy of the frontotemporal cerebral cortex and ventricular enlargement (Fig. 1f).
Prion protein gene testing was performed in patients 1 and 2,1, and genetic CJD (gCJD) was diagnosed based on the presence of five identical insertion mutations in the OPR region in both cases: (R1-R2-R2-R3g-R2-R3g-R2-R2-R3-R4), (NC_000020.11:g.4699465_4699466ins[GCAGCCTCATGGTGGTGGCTGGGGGCAGCCCCAT;4699380_4699465]). Patient 1 was found to be heterozygous for methionine-valine at codon 129 and homozygous for glutamine at codon 219, and the 5-OPRI mutation was found on the M allele side. Patient 2 was found to be homozygous for methionine at codon 129 and glutamine at codon 219.
Our literature search found 20 patients with reported 5-OPRI mutations across nine families in six research articles, including this report (Table 1)1,3,4,5,6,7. Pathological diagnosis was performed on 10 patients. Only two families had common mutations (KE and NI). The mean age at onset was 45 years (standard error, 2.53), and only four patients had a total disease duration of <2 years. Progressive dementia was present in all cases, and descriptions of findings suspicious for frontotemporal dementia (e.g., behavioral disinhibition; apathy or behavioral inertia; loss of sympathy or empathy; perseverative, stereotyped or compulsive/ritualistic behavior; hyperorality and dietary changes; and frontal release signs) were found in 14 patients. MRI was performed in 12 patients, but DWI and fluid-attenuated inversion recovery (FLAIR) imaging, which are typically helpful in diagnosing CJD, were described in only four patients, and none of them showed abnormal signals. Electroencephalography was performed in 10 patients, and PSD was noted in only two. The 14-3-3 protein levels were measured in five patients, one of whom tested positive.
The two reported cases showed no cortical hyperintensities on DWI/FLAIR images, no PSD on EEG, and negative 14-3-3 protein and RT-QUIC. According to our literature review, gCJD caused by 5-OPRI mutations has a markedly younger onset and a longer disease duration than sCJD. Many patients lack myoclonus and exhibit symptoms suggesting the behavioral variant of frontotemporal dementia8. There is no typical PSD on EEG, and there are no cortical hyperintensities on DWI or FLAIR brain MRI, although this has been confirmed only in recent reports. Since the 5-OPRI mutation is capable of infecting other organisms, as revealed in experimental infection studies, the recommendation is to pursue a genetic diagnosis of gCJD aggressively, even if the case is not typical of sCJD.
Patients with gCJD due to 5-OPRI mutations show the behavioral variant of frontotemporal dementia from a young age, do not present with myoclonus, and have no obvious abnormalities in either EEG or cortical signals on MRI. These diseases do not meet the usual diagnostic criteria for prion diseases, and the utility of genetic diagnosis must be emphasized.
References
Goldfarb, L. G. et al. Transmissible familial Creutzfeldt-Jakob disease associated with five, seven, and eight extra octapeptide coding repeats in the PRNP gene. Proc. Natl. Acad. Sci. USA 88, 10926–10930 (1991).
Hiraga, C., Kobayashi, A. & Kitamoto, T. The number of octapeptide repeat affects the expression and conversion of prion protein. Biochem. Biophys. Res. Commun. 382, 715–719 (2009).
Cochran, E. J. et al. Familial Creutzfeldt-Jakob disease with a five-repeat octapeptide insert mutation. Neurology 47, 727–733 (1996).
Skworc, K. H. et al. Familial Creutzfeldt-Jakob disease with a novel 120-bp insertion in the prion protein gene. Ann. Neurol. 46, 693–700 (1999).
Beck, G. et al. A case with a 120 base pair insertional mutation in the prion protein gene: the first case in Japan. J. Neurol. Neurosurg. Psychiatry 76, 756–757 (2005).
Mead, S. et al. Inherited prion disease with 5-OPRI: phenotype modification by repeat length and codon 129. Neurology 69, 730–738 (2007).
Jansen, C. et al. Inherited Creutzfeldt-Jakob disease in a Dutch patient with a novel five octapeptide repeat insertion and unusual cerebellar morphology. J. Neurol. Neurosurg. Psychiatry 80, 1386–1389 (2009).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioral variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Acknowledgements
This work was supported by a grant-in-aid from the Research Committee of Prion Disease Surveillance, Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Contributions
S.H., F.M., and I.Y. conceived the idea of the study. KS conducted biochemical analysis. TK conducted genetic analysis. SH and IT drafted the original manuscript. HM and IY supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
K.S., T.K., H.M., F.M., and I.Y. received a grant-in-aid from the Research Committee of Prion Disease Surveillance, Ministry of Health, Labor and Welfare of Japan. The other authors declare that they have no conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamada, S., Takahashi-Iwata, I., Satoh, K. et al. Genetic Creutzfeldt‒Jakob disease with 5-octapeptide repeats presented as frontotemporal dementia. Hum Genome Var 10, 10 (2023). https://doi.org/10.1038/s41439-023-00237-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-023-00237-w