Abstract
We report the first case of Waardenburg syndrome type 4C and Kallmann syndrome in the same person. The patient, a Japanese girl, presented with bilateral iris depigmentation, bilateral sensorineural hearing loss, Hirschsprung disease, hypogonadotropic hypogonadism, and anosmia. We identified a novel SOX10 variant, c.124delC, p.Leu42Cysfs*67.
Similar content being viewed by others
Waardenburg syndrome (WS), a rare autosomal dominant disorder, is characterized by sensorineural hearing loss and pigmentation abnormalities of the hair, irises and skin1. WS is classified into four subtypes, WS1, WS2, WS3, and WS4. WS4 is defined as a complication of Hirschsprung disease. WS4 is genetically heterogeneous, with three causative genes identified2. WS, type 4C (WS4C, OMIM#613266) is caused by heterozygous mutation in the SOX10 gene, whereas WS, type 4A (WS4A, OMIM#277580) and WS, type 4B (WS4B, OMIM#613265) are caused by mutation of EDNRB and EDN3, respectively. SOX10 belongs to the SOX family, the members of which have a high mobility group (HMG) DNA binding domain. SOX10 plays a major role in the development and migration of neural crest cells, which can differentiate into melanocytes, olfactory ensheathing cells, and enteric ganglia neurons1,2,3,4,5.
Kallmann syndrome (KS) is a clinically and genetically heterogeneous disorder defined by hypogonadotropic hypogonadism (HH) and olfactory dysfunction. KS is occasionally associated with hearing loss, which occurs in approximately 5% of patients6. Several genetic variants have been linked to the etiology of KS with hearing loss, including KAL1, FGFR1, FGF8, IL17RD, and CHD77,8,9,10. Recently, variants in SOX10 have been identified in a few KS patients with hearing loss11.
Here, we report for the first time a case with both WS4C and KS due to a novel SOX10 variant. The proband was referred to our outpatient clinic at 14.9 years of age for lack of pubertal development. She was born to nonconsanguineous Japanese parents at 39 weeks and 6 days of gestation. The pregnancy and delivery were uncomplicated. Her birth weight and length were 2890 g (−0.6 SD) and 47.0 cm (−1.3 SD), respectively. Shortly after birth, bilateral iris depigmentation was noted (Fig. 1a, b). At 24 h of life, she exhibited abdominal distention, vomiting, and delayed meconium excretion. The intraoperative findings showed caliber changes. The rectal mucosal biopsy sample was positive for acetylcholinesterase staining, which confirmed the diagnosis of Hirschsprung disease. The newborn hearing screening test produced a ‘refer’ result, and auditory brainstem response (ABR) revealed congenital bilateral sensorineural hearing loss. Thus, she was clinically diagnosed with WS4. Her mental development was normal. On the first visit at 14.9 years of age, her height was 142.2 cm (−2.79 SD). She showed no pubic hair or breast development (Tanner stage 1). Endocrinological evaluation showed poor gonadotropin response after gonadotropin-releasing hormone stimulation; basal and peak LH values of <0.2 mIU/ml (reference range: 0.4–4.1) and 3.1 mIU/ml (reference range: 8.5–15.5), respectively; basal and peak FSH values of 1.1 mIU/ml (reference range: 4.8–10.4) and 8.5 mIU/ml (reference range: 8.3–20.0), respectively, and a low plasma estradiol level (10.0 pg/ml; reference range, 11.0–172). The secretion of the other pituitary hormones was normal. The chromosome G-banding analysis showed 46,XX. Intravenous olfactometry (alinamin test) induced no response, indicating anosmia. Brain magnetic resonance imaging (MRI) revealed olfactory bulb agenesis but no abnormalities in the hypothalamus or pituitary (Fig. 1c, d). Her parents and older brother had normal height and age-appropriate pubertal development. Finally, this case was diagnosed as having WS4 and KS.
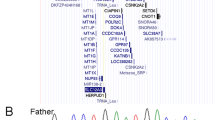
We received approval for the genetic study from the Ethics Committee of Ehime University Graduate School of Medicine and the Ethics Committee of Keio University School of Medicine. After obtaining written informed consent from her parents, we extracted genomic DNA from peripheral blood samples from the patient. We analyzed major causative genes and possible causative genes for KS and/or HH, i.e., AXL, CCDC141, CHD7, DMXL2, DUSP6, FEZF1, FGF8, FGF17, FGFR1, FLRT3, FSHB, GH1, GHR, GHRH, GHRHR, GHSR, GLI2, GNRH1, GNRHR, GPR161, HESX1, HS6ST1, IGF1, IGF1R, IL17RD, KAL1, KISS1, KISS1R, LHB, LHX3, LHX4, LEP, LEPR, MKRN3, NELF, OTUD4, OTX2, PAX6, PCSK1, PGM1, PNPLA6, POLR3A, POLR3B, POU1F1, PROK2, PROKR2, PROP1, RNF216, SEMA3A, SEMA7A, SIX3, SIX6, SOX2, SOX10, SPRY4, STAT5B, STUB1, TAC3, TACR3, TBX19, and TUBB3 using next generation sequencing on a MiSeq instrument (Illumina, San Diego, CA, USA), according to the SureSelect protocol (Agilent Technologies, Santa Clara, CA, USA). We identified a novel heterozygous variant, c.124delC, p.Leu42Cysfs*67, in exon 3 in SOX10 (Fig. 2). This variant was not found in the Human Genetic Variation Database (http://www.hgvd.genome.med.kyoto-u.ac.jp/), the gnomAD browser (https://gnomad.broadinstitute.org/), or the 1000 Genome Browser (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/). No further pathogenic variant was identified in the other tested genes.
This is the first case of a patient with both WS4C and KS. We, however, cannot deny the possibility that previous reports of WS4C have not been well investigated for gonadal function. When the patient is diagnosed with WS4C in infancy, it is impossible to determine whether the patient has hypogonadism unless he or she is followed until pubertal age.
A novel heterozygous variant, c.124delC, p.Leu42Cysfs*67, in SOX10 was responsible for the WS4C and KS in this case. This frameshift mutation results in a stop codon, theoretically leading to nonsense-mediated messenger RNA decay, and probably causes haploinsufficiency of SOX10. The nonsense mutation p.Glu189* in exon 4 in SOX10 has been reported in a patient with WS412 and in a patient with KS, WS1, and hyperthyroidism13. Notably, Inoue et al.14 reported that the p.Glu189* and p.Tyr207* mutations in exon 4 in SOX10 showed nonsense-mediated messenger RNA decay. Izumi et al.15 determined that the p.Pro169fs*117 mutation in SOX10, which was found in a female patient diagnosed with KS and WS2, was haploinsufficient by an in vitro assay. Amato et al.16 reported a large heterozygous deletion involving the entire coding region of SOX10 in a woman with KS and bilateral sensorineural hearing loss. These findings together with the present case suggest that SOX10 haploinsufficiency has wide clinical variation.
In summary, we identified a novel SOX10 variant in a Japanese girl first diagnosed with WS4C and KS. Patients with WS4C due to a SOX10 variant should be examined and followed for clinical features of KS.
HGV Database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.2906.
References
Pingault, V. et al. Review and update of mutations causing Waardenburg syndrome. Hum. Mutat. 31, 391–406 (2010).
Chaoui, A. et al. Identification and functional analysis of SOX10 missense mutations in different subtypes of Waardenburg syndrome. Hum. Mutat. 32, 1436–1449 (2011).
Hou, L., Arnheiter, H. & Pavan, W. J. Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc. Natl Acad. Sci. USA 103, 9081–9085 (2006).
Barraud, P., St John, J. A., Stolt, C. C., Wegner, M. & Baker, C. V. Olfactory ensheathing glia are required for embryonic olfactory axon targeting and the migration of gonadotropin-releasing hormone neurons. Biol. Open 2, 750–759 (2013).
Kuhlbrodt, K., Herbarth, B., Sock, E., Hermans-Borgmeyer, I. & Wegner, M. SOX10, a novel transcriptional modulator in glial cells. J. Neurosci. 18, 237–250 (1998).
Quinton, R. et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin. Endocrinol. (Oxf.) 55, 163–174 (2001).
Marlin, S. et al. Discovery of a large deletion of KAL1 in 2 deaf brothers. Otol. Neurotol. 34, 1590–1594 (2013).
Costa-Barbosa, F. A. et al. Prioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypes. J. Clin. Endocrinol. Metab. 98, E943–E953 (2013).
Suzuki, E. et al. De novo frameshift mutation in fibroblast growth factor 8 in a male patient with gonadotropin deficiency. Horm. Res Paediatr. 81, 139–144 (2014).
Miraoui, H. et al. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am. J. Hum. Genet. 92, 725–743 (2013).
Pingault, V. et al. Loss-of-function mutations in SOX10 cause Kallmann syndrome with deafness. Am. J. Hum. Genet. 92, 707–724 (2013).
Pingault, V. et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 18, 171–173 (1998).
Wang, F., Zhao, S., Xie, Y., Yang, W. & Mo, Z. De novo SOX10 nonsense mutation in a patient with Kallmann syndrome, deafness, iris hypopigmentation, and hyperthyroidism. Ann. Clin. Lab Sci. 48, 248–252 (2018).
Inoue, K. et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat. Genet. 36, 361–369 (2004).
Izumi, Y. et al. Hypogonadotropic hypogonadism in a female patient previously diagnosed as having Waardenburg syndrome due to a SOX10 mutation. Endocrine 49, 553–556 (2015).
Amato, L. G. L. et al. New genetic findings in a large cohort of congenital hypogonadotropic hypogonadism. Eur. J. Endocrinol. 181, 103–119 (2019).
Acknowledgements
The authors thank the patient and her family for their participation in this study.
Author information
Authors and Affiliations
Contributions
J.H., F.O., and Y.S. managed the patient and prepared the manuscript. M.H., H.S., and T.H. performed the molecular analyses. K.T., H.H., T.H., and M.E. supported the manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Tomonobu Hasegawa has the following financial relationships to disclose: Research funding from Novo Nordisk Pharma Ltd. and JCR Pharmaceuticals Co., Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamada, J., Ochi, F., Sei, Y. et al. A novel SOX10 variant in a Japanese girl with Waardenburg syndrome type 4C and Kallmann syndrome. Hum Genome Var 7, 30 (2020). https://doi.org/10.1038/s41439-020-00118-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-020-00118-6