Abstract
Red bayberry (Morella rubra) is an evergreen fruit tree found in southern China whose whole-genome sequence has recently been published. We updated the linkage map of the species by adding 118 SSR markers and the female-specific marker MrFT2_BD-SEX. The integrated map included eight linkage groups and spanned 491 cM. Eleven sex-associated markers were identified, six of which were located in linkage group 8, in agreement with the previously reported location of the sex-determining region. The MrFT2_BD-SEX marker was genotyped in 203 cultivated accessions. Among the females of the accessions, we found two female-specific alleles, designated W-b (151 bp) and W-d (129 bp). We previously found that ‘Dongkui’, a female cultivar, could produce viable pollen (we refer to such plants ‘Dongkui-male’) and serve as the paternal parent in crosses. The genotypes of the MrFT2_BD-SEX marker were W-b/Z in ‘Biqi’ and W-d/Z in ‘Dongkui-male’. The progeny of a cross between these parents produced a 3:1 female (W-) to male (ZZ) ratio and the expected 1:1:1:1 ratio of W-b/W-d: W-b/Z: W-d/Z: Z/Z. In addition, the flowering and fruiting phenotypes of all the F1 progeny fit their genotypes. Our results confirm the existence of ZW sex determination and show that the female phenotype is controlled by a single dominant locus (W) in a small genomic region (59 kb and less than 3.3 cM). Furthermore, we have produced a homozygous “super female” (WW) that should produce all-female offspring in the F2 generation, providing a foundation for commercial use and presenting great potential for use in modern breeding programs.
Similar content being viewed by others
Introduction
Sexual systems in flowering plants are diverse, and ~6% of species are dioecious, with separate female and male plants1. Sex is often genetically determined, sometimes by sex chromosomes2. Sex chromosomes are typically thought to evolve from specific pairs of autosomes that may subsequently increase or decrease in size to form homomorphic or heteromorphic sex chromosomes3. Unlike the sex chromosomes of many animals, plants usually have homomorphic sex chromosomes that are not cytologically distinguishable in early stages of sex chromosome evolution, as demonstrated in kiwifruit (Actinidia chinensis)4, wild strawberry (Fragaria virginiana)5, willow (Salix viminalis)6, and garden asparagus (Asparagus officinalis)7. They can be divided into XY systems (homozygous XX females and heterozygous XY males), as observed in papaya (Carica papaya)8, persimmon (Diospyros lotus)9, date palm (Phoenix dactylifera)10, and poplar (Populus trichocarpa)11, and ZW systems (females heterozygous ZW and male homozygous ZZ), as observed in willow (Salix viminalis)12 and wild strawberry (Fragaria virginiana)5.
In several plants with homomorphic sex chromosomes, it has been reported that sex determination is controlled by a small region that prevents recombination around the sex-determining locus. For example, wild strawberry (Fragaria virginiana) has two sex-determining loci, showing recessive male sterility (g) and dominant female fertility (A), with a 280 kb sex determination region located in linkage group 65. In asparagus (Asparagus officinalis), sex determination is controlled by a single locus, M, on chromosome 5, including two tightly linked genes: the female suppressor (F) and male activator (M) 13.
Sex determination in plants is a universal biological process and is very important in agriculture, horticulture, and environmental protection14, because female and male plants present different value, especially in early stage identification in perennial species. Red bayberry (Morella rubra, formerly Myrica rubra) is usually dioecious, with female plants being used for commercial fruit production and males for pollination. However, it has a long juvenile period, making it time-consuming to identify the plants’ sexes. As red bayberry is an important fruit in southern China, it is important to study the sex-determination mechanism of this species. Using the assembled genomes of one female and one male individual, we previously proposed a ZW model of sex determination and identified a small (59 Kb) female-specific region at the distal end of chromosome 815. However, the ZW model has not been verified, and how the sex-linked region is inherited remains largely unknown.
‘Biqi’ and ‘Dongkui’ are two economically important, genetically distinct female red bayberry cultivars16,17. In 2011, we identified rare male catkins and viable pollen in the ‘Dongkui’ cultivar after applying a vigor control regulator (containing uniconazole) to plants in July and August18 and designated the source pollen as ‘Dongkui-male’. F1 progeny were successfully obtained from the first cross between ‘Biqi’ and ‘Dongkui-male’19.
The availability of F1 progeny allowed us to add codominant SSR markers to the SNP genetic linkage map used for genome assembly and genetic analysis. Segregation in the F1 population revealed a dominant female-specific W-locus controlling sex determination, confirming the ZW sex chromosome system of red bayberry. The F1 population also produced homozygous “super female” (WW) individuals, which could theoretically generate all-female offspring in F2 for further study and commercial breeding.
Results
Linkage map construction
To construct a genetic linkage map, 95 F1 progeny and 127 SSR markers that were heterozygous in ‘Biqi’, ‘Dongkui-male’ or both were selected. The MrFT2_BD-SEX marker, which was developed from the female-specific region15 and exhibited different fragments between the parents ‘Biqi’ and ‘Dongkui-male’, was also included. Based on the published biparental genetic linkage map of red bayberry15, we then estimated a new integrated high-density SSR-SNP linkage map (Fig. S1) with 3,073 SNP and 118 SSR markers and MrFT2_BD-SEX, while the nine unmapped SSR markers presented unclear genotypes or distorted segregation ratios. The final map spanned 491 cM with an average marker interval of 0.15 cM (Table 1).
We screened all SSR markers from the high-density SSR-SNP map (Fig. S1) to obtain an SSR-based genetic linkage map (Fig. 1) containing 118 SSR markers and the MrFT2_BD-SEX marker. Among the mapped markers, 45 (37.8%) segregated in female meiosis (with the parental genotypes lm × ll, where different letters denote distinct alleles), and 25 (21.0%) segregated in male meiosis (nn × np), while 43 (36.1%) fully informative markers were heterozygous in both parents (12 with four distinct alleles, ab×cd type, and 31 with 3 alleles, ef × eg), and 6 (5.0%) were partially informative in both parents (hk × hk). Searching for markers near the female-specific marker MrFT2_BD-SEX yielded two flanking SSR markers, ZJU254 and ZJU079, at distances of 0.7 cM and 3.3 cM from the MrFT2_BD-SEX marker, respectively (Fig. 1). Two SSR markers that were previously predicated as sex-associated, ZJU062 and ZJU13017, were located in LG7 and LG3, respectively (Fig. 1).
We next obtained the physical linkage map by aligning the PCR product sequences of the mapped markers in an SSR-based genetic linkage map to the chromosome sequences (Fig. S2). One hundred and eleven markers were anchored to the assembled genome sequences, among which three were tandemly duplicated (ZJU247, ZJU238, and ZJU021), one was duplicated in a different region of the same linkage group (my0889), and two were duplicated in different linkage groups (ZJU202 and ZJU137). Among the remaining eight SSR markers, five matched unmapped scaffolds, and three did not match any sequences.
SSR marker association analysis in the dioecious population
Based on the SSR-based linkage map, 93 SSR markers and MrFT2_BD-SEX were used to evaluate the association between the markers and the sex phenotype in 203 accessions, including 95 of the main cultivated varieties (females), 107 males, and one monoecious individual, collected from Zhejiang, Jiangsu, Fujian, Hunan, Guangxi, and Guizhou Provinces (Table S1). Twelve accessions (four females and eight males) from Hunan Province and 39 SSR markers were added compared with our earlier report17.
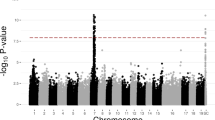
Structural analysis divided the 203 accessions into two groups, as the lnP (K) function increased without a turning point, while the delta K (ΔK) function presented a clear inflexion point at K = 2. We analyzed the correlation between the markers and sex traits by combining the population structure, genotype, and sex phenotype data using the general linear model (GLM) method. The results showed that 11 markers were associated with the sex phenotype, and six of them were located in LG8 (Table S2). The highest sex phenotype variation (R2) value, of 0.97, was obtained for the MrFT2_BD-SEX marker (Fig. 2 and Table 2); this marker was amplified only in the female accessions, generating products of 129 bp and 151 bp, with allele frequencies of 0.484 and 0.516, respectively (Table S2). The MrFT2_BD-SEX marker therefore accurately identifies the sexes of cultivated accessions, which implies that the marker is completely sex linked in the cultivated red bayberry accessions. Based on the population structure17, we found the 129 bp allele in most of the ‘Fenhong’ and ‘Dongkui’ subgroups and the 151 bp type in the ‘Biqi’ subgroup (Table S1) and the monoecious individual. For the ZJU079 marker, which was the marker closest to MrFT2_BD-SEX in linkage group 8 (3.3 cM) (Fig. 2 and Table 2), the most common alleles in males were 124 and 130 bp, versus 128 and 134 bp in females (Table S2).
The total genetic distance, covering eight linkage groups, is indicated on the X-axis. The explained phenotypic variation between the markers and the sex population is shown on the Y-axis. The six potential sex-associated markers are indicated in red in LG8. The sex-associated markers are indicated by arrows
Sex phenotype in the F1 mapping population
The F1 seedlings were planted in 2013 and 2015. Sixteen individuals flowered in 2019, and their sex phenotypes were determined from their catkins and fruits (Fig. 3b and Table S3). The F1 seedlings were also genotyped for the MrFT2_BD-SEX marker, which identified 70 individuals as females (129 bp, 151 bp, or heterozygous for both these alleles) and 25 as males (no amplification), in agreement with the Mendelian segregation ratio of 3:1 expected under the assumption that the female is heterozygous ZW (Chi-square p value 0.77). The sex phenotypes of the sixteen offspring that flowered were consistent with the MrFT2_BD-SEX amplification results (Table S3, Fig. 3b).
a The sex-determination model of ZW sex chromosomes in red bayberry. The female-specific region (FSR) within the W chromosome is indicated in purple, the FSR flanking region is indicated in pink, and the autosomal region is indicated in green. b The genotypes of the parents are predicted to be W-b/Z (‘Biqi’) and W-d/Z (‘Dongkui-male’). The genotypes of the F1 progeny are segregated into four types: W-b/W-d, W-b/Z, W-d/Z, and Z/Z, and the corresponding individuals' sex phenotype are shown in photos
According to the ZW model, the genotypes of the ‘Biqi’ and ‘Dongkui-male’ parents at the MrFT2_BD-SEX marker are W-b/Z (151 bp) and W-d/Z (129 bp) (Fig. 3a), respectively. In the ZW × ZW cross population, we observed the expected four genotypes: (1) 151 bp alone (W-b/Z, 18 individuals); (2) 129 bp alone (W-d/Z, 33 individuals); (3) both 151 bp and 129 bp (W-b/W-d, 19 individuals); and (4) Z/Z males with no detectable marker allele (25 individuals). The numbers of individuals of the different genotypes fit the expected 1:1:1:1 ratio (Chi-square test p = 0.11 > 0.05). The viable W-b/W-d genotype indicated that the WW genotype could represent a “super female” population, which should be useful for producing all-female F2 progeny.
Around the sex marker MrFT2_BD-SEX, one flanking marker, ZJU079, was heterozygous in ‘Biqi’ (128/124 bp) and ‘Dongkui-male’ (132/124 bp), with 128 bp and 132 bp alleles in the W region (since they were transmitted to most female progeny) and a 124 bp allele in the Z region. The other flanking marker, ZJU254, was heterozygous in ‘Biqi’ (195/201 bp) but homozygous in ‘Dongkui-male’ (197/197 bp).
Discussion
The female phenotype of red bayberry is controlled by a dominant locus in linkage group 8
Understanding sex-determination mechanisms is essential to illustrate the evolution of sex chromosomes. Red bayberry is normally dioecious with a few monecious individuals, and it has homomorphic ZW sex chromosomes, as observed in wild strawberry5 and poplar12. In this study, the “super-female” genotype (homozygote WW) was viable in the F1 progeny, unlike the situation in papaya (Carica papaya), where the YY genotype is lethal20. To the best of our knowledge, the viability of WW genotypes was first reported in fruit trees. These results suggest that red bayberry represents stage two of sex chromosome evolution, with a small W-linked region that evolved recently enough for genetic degeneration not to have become advanced1. The WW genotype provides valuable material that may contribute to the assembly of the W region and the determination of the boundary between the Z- and W-linked regions of this chromosome. Homozygous “super female” (WW) plants will also be useful for further breeding programs with the aim of producing all-female F2 progeny for fruit production.
In the F1 progeny and 203 cultivated accessions, MrFT2_BD-SEX was inferred to be a completely sex-linked marker, as it accurately identified the sex of red bayberry. Based on the SSR-based linkage map (Fig. 1), two SSR markers flanking MrFT2_BD-SEX in the F1 seedlings showed that ZJU079 (3.3 cM) might be partially sex linked according to Mendelian segregation and the Chi-square test (p = 0.27), while ZJU254 (0.7 cM) might not be sex linked because of the homozygous fragment of ‘Dongkui-male’, although it was very close to the sex marker MrFT2_BD-SEX (Table S3). This result is consistent with that of GLM analysis in the 203 cultivated accessions. In our study, the physical distance between MrFT2_BD-SEX and ZJU079 was found to be 1.0 Mb (Fig. S1), which is larger than the female-specific region (59 Kb) reported before15. This shows that ZJU079 is sex-related and narrows the female-specific region to less than 3.3 cM. This result is consistent with the homomorphic sex chromosomes of this plant, and it suggests that recombination has not been suppressed and occurs in genome regions close to the sex-determination locus6. The complete W-linked region of red bayberry chromosome 8 appears to be physically small (59 kb and less than 3.3 cM on chromosome 8), even smaller than the regions recently identified in several other dioecious plants, including willow (840 kb on chromosome 15)6, wild strawberry (280 kb in linkage group 6), and kiwifruit (1 Mb on chromosome 25)21.
In red bayberry, the genetic background of cultivated accessions is complex, with the female plants having been selected over many years for better fruit quality, while the male plants are basically wild accessions due to their low commercial value. Without linkage map information, the allele frequencies in the two sex populations identified two markers, ZJU130 and ZJU062, that were moderately associated with sex17, which have now been located in linkage groups 3 and 7, respectively, implying that they are accompanying sex stratification factors rather than sex-determining factors. In this study, GLM analysis identified ten SSR markers that might be associated with the sex phenotype, including ZJU062 from a previous report17. Five SSR markers were located in LG8, among which two (ZJU079 and ZJU060) were located very close to MrFT2_BD-SEX, and the other three (ZJU021, my0427, and my0972) were located at the beginning of LG8, which might be related to homologues of female-specific genes. Markers located in other linkage groups may be related to the evolution of other traits in male and female plants, such as flowering, variety selection and fruit quality.
It is interesting that the female cultivars occasionally exhibit mutated or induced male branches in nature. In our previous study, we found two types of sex-changing branches in red bayberry. One (‘Biqi-male’), from the ‘Biqi’ cultivar, was permanent, as some branches bore male flowers and remained unchanged in the following years; the other (‘Dongkui-male’) showed induced viable pollen in the ‘Dongkui’ cultivar and resulted in occasional male flowers on female branches, which returned to an all-female phenotype in the following years. For both of these types of sex-changing branches, the viable pollen presented the same genotype as the corresponding female individuals (Table S1) according to the MrFT2_BD-SEX marker. It is unknown why males can show amplification of the same genotypes as female individuals in red bayberry. We suggest that the ZW females might be controlled by two sex-determining genes within the young sex chromosome22 and might have undergone the loss of a W-linked male-suppressing factor, similar to the suppressor of feminization (SyGI) and maintenance of male (M) (FrBy) in kiwifruit, leading to a two-factor sex-determination system23. Similarly, mutant XY males in Silene latifolia have lost a female-suppressing factor and become functional hermaphrodites24. Considering the male-specific DNA methylation patterns in poplar25 and the role of microRNAs as the key sex regulators in persimmon9, it is also reasonable to speculate that epigenetic regulation mechanisms such as DNA methylation, histone modifications and microRNAs might also contribute to sex determination, in addition to genetic regulation in red bayberry.
The genetic linkage map gives us a better understanding of sex determination in red bayberry
De novo genome sequencing has had a profound impact on genetics and various applications26,27. Red bayberry is a perennial woody plant with a long juvenile stage. In this study, we constructed an integrated SSR-SNP linkage map with an average marker distance of 0.15 cM. This SSR-SNP genetic map provides a powerful tool for locating sex-related markers and identifying the sex-determination mechanisms of cultivated accessions and cross populations. The map will also serve as a reference to align different mapping populations for genome assembly and lays the foundation for the genetic and genomic analysis of traits related to disease resistance, productivity and fruit quality. The mapped SSR markers can also be valuable for identifying cultivars, evaluating genetic diversity, and studying the relationships and origins of cultivars.
According to the physical map (Fig. S2), certain SSR markers were distributed in multiple linkage groups, which might be due to repeat sequences in the genome, as the red bayberry genome harbors 36.4% repetitive sequences15, indicating that duplications are not implausible. SSR duplication has been reported in other fruit, such as strawberry28, jujube29, pear30, and citrus31. The comparison of the SSR-based linkage map and physical map revealed some differences in the marker order, which may be due to the lack of one-to-one correspondence of the markers to the scaffolds15 and the high number of repetitive sequences affecting the quality of the assembly. The SSR markers matched unmapped scaffolds or did not match any sequences, probably because the anchored physical linkage map covered 90% of the assembled genome and 87% of the whole genome15. Some order issues remain, and 10% of the genome sequence needs to be corrected and assembled.
The cultivated female varieties were divided into two groups according to the MrFT2_M2-SEX marker, with the first consisting of the ‘Biqi’ series, while the second consists of the ‘Dongkui’ and ‘Fenhong’ cultivars (Table S1). This suggests that cultivated varieties might have evolved from two progenitors. The difference between the two genotypes was a difference in fragment size, with ‘Biqi’ presenting a larger fragment than ‘Dongkui’. This indel could have important consequences for the evolution of plant genomes because variations in self-fertilization in plant species lead to differences in the heterozygosity of alleles32.
In conclusion, we developed a new SSR-SNP genetic linkage map for red bayberry. From the map, we identified a sex-related SSR marker, ZJU079, and confirmed that the sex-determination region is very short (59 kb and less than 3.3 cM) in linkage group 8. Our results confirm that the sex mechanism is genetically controlled by a single dominant female-specific locus (W).
Materials and methods
Plant materials and DNA extraction
An F1 population was derived from hybrid progeny of two cultivars, ‘Biqi’ and ‘Dongkui-male’19, in 2011 and 2013. From this population, 95 samples were selected for mapping, including 77 individuals obtained from the 2011 crossing population reported previously19 and 18 generated in 2013. Young leaves of the hybrids were collected from Yuyao, Zhejiang Province, China, frozen in liquid nitrogen and stored at −40 °C.
Genomic DNA was extracted from these samples using the modified cetyltrimethylammonium bromide (CTAB) method33 and then diluted to 30 ng µl−1 for PCR amplification.
SSR marker amplification
The SSR markers were developed from WGS sequences16,34,35 and the M. rubra EST database36. The origin of the polymorphic markers is given in Table S4. A three-primer strategy was used for the PCR amplification of SSR markers, involving a regular reverse primer, a forward primer with an M13 tail at the 5’ end, and a forward primer including an M13 tail with one of the following fluorescence labels at the 5’ end: FAM (blue), HEX (green), NED (yellow), or PET (red) (Invitrogen, Shanghai, China). The MrFT2_BD-SEX marker was developed from the DNA sequence of the female-specific putative gene MrFT2, and its fragment size differs between ‘Biqi’ and ‘Dongkui-male’. The forward primer was FAM 5′-GCGGTATAGTAATCAGGATTCCAT-3′, while the reverse primer was 5′-GGGTTCCATCATAAGGACATTTGT-3′. We first chose two parents and six of their progeny to examine the polymorphism of SSR markers. Eight polymorphic SSR markers (Invitrogen, Shanghai, China) were selected for true hybrid identification19. The PCR system and procedure used for SSR marker analysis were as described by Terakawa et al.34 and Jiao et al.16. The 15 µl reaction mixtures contained 5 pmol reverse primer, 1 pmol M13-tailed forward primer, 4 pmol M13-labeled fluorescent primer, and PremixTaqTM Hot Start Version (TaKaRa, Dalian, China) in an amount according to the manufacturer’s instructions, and 30 ng genomic DNA template. The two-step PCR amplification procedure was as follows: 94 °C for 5 min, 30 cycles of 30 s at 94 °C, 30 s at 58 °C, and 30 s at 72 °C, and then eight cycles of 30 s at 94 °C, 30 s at 53 °C, and 30 s at 72 °C, with a final extension at 72 °C for 10 min. The PCR mixture for MrFT2_BD-SEX was the same as for the SSR markers but without an M13-tailed forward primer, and the PCR amplification procedure was as follows: 94 °C for 5 min, 35 cycles of 30 s at 94 °C, 30 s at 58 °C, 30 s at 72 °C, and a final extension at 72 °C for 10 min. The PCR products with different sizes and fluorescence labels were mixed with the GeneScan LIZ500 standard (Applied Biosystems) and detected in an ABI3130 DNA analyzer with the allele size analyzed by GeneMapper v4.0 software (Applied Biosystems, CA).
Linkage map construction
The genotype data of the polymorphic SSR markers and MrFT2_BD-SEX were scored by JoinMap 4.137. The population type was CP, a population resulting from a cross between two heterozygous diploid parents and possibly an unknown linkage phase. The segregation type codes were ab × cd, ef × eg, hk × hk, lm × ll, and nn × np. According to the expected Mendelian ratio, the segregation of markers was tested to identify distorted markers according to the Chi-square test, and these markers were excluded. Markers were grouped using a threshold independence logarithm of odds (LOD) value of 7.0, and eight linkage groups were selected on the basis of the known chromosome number according to a cytological study in Myricaceae38, in accordance with the order of chromosomes published by Jia et al.15. The regression mapping algorithm and Kosambi’s mapping function39 were applied for marker ordering in the group, and the map distances were generated in centimorgans. MapChart40 was used to draw the linkage maps.
General linear model analysis of two sex populations
STRUCTURE v.2.041 was applied to 203 accessions to infer the population structure using a 100,000 times burn-in period and 100,000 MCMC iterations. The test K values ranged from 1 to 10 with ten independent runs. STRUCTURE HARVESTER (http://taylor0.biology.ucla.edu/structureHarvester/)42 was used to generate the Q-matrix under the most likely K value according to the delta K (ΔK) value. The associations between the genotypes and phenotypes of the 203 accessions were evaluated with the general linear model (GLM) using TASSEL version 2.1.0.
References
Ming, R. et al. Sex chromosomes in land plants. Annu Rev. Plant Biol. 62, 485–514 (2011).
Bachtrog, D. et al. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 (2014).
Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 88, 94–101 (2002).
Akagi, T. et al. A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit. Plant Cell 30, 780–795 (2018).
Tennessen, J. et al. Homomorphic ZW chromosomes in a wild strawberry show distinctive recombination heterogeneity but a small sex-determining region. N. Phytol. 211, 1412–1423 (2016).
Pucholt, P. et al. Recent sex chromosome divergence despite ancient dioecy in the Willow Salix viminalis. Mol. Biol. Evol. 34, 1991–2001 (2017).
Telgmann-Rauber, A. et al. Genetic and physical maps around the sex-determining M-locus of the dioecious plant asparagus. Mol. Genet. Genomics 278, 221–234 (2007).
Wang, J. P. et al. Sequencing papaya X and Y-h chromosomes reveals molecular basis of incipient sex chromosome evolution. Proc. Natl Acad. Sci. USA 109, 13710–13715 (2012).
Akagi, T. et al. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science 346, 646–650 (2014).
Cherif, E. et al. Male-specific DNA markers provide genetic evidence of an XY chromosome system, a recombination arrest and allow the tracing of paternal lineages in date palm. N. Phytol. 197, 409–415 (2013).
Geraldes, A. et al. Recent Y chromosome divergence despite ancient origin of dioecy in poplars (Populus). Mol. Ecol. 24, 3243–3256 (2015).
Pucholt, P. et al. Single locus sex determination and female heterogamety in the basket willow (Salix viminalis L.). Heredity (Edinb.) 114, 575–583 (2015).
Moreno, R. et al. Integration of genetic and cytogenetic maps and identification of sex chromosome in garden asparagus (Asparagus officinalis L.). Front. Plant. Sci. https://doi.org/10.3389/fpls.2018.01068 (2018).
Milewicz, M. & Sawicki, J. Sex-linked markers in dioecious plants. Plant Omics 6, 144–149 (2013).
Jia, H. M. et al. The red bayberry genome and genetic basis of sex determination. Plant Biotechnol. J. 17, 397–409 (2019).
Jiao, Y. et al. Development of simple sequence repeat (SSR) markers from a genome survey of Chinese bayberry (Myrica rubra). BMC Genomics 13, 201 (2012).
Jia, H. M. et al. Genetic diversity of male and female Chinese bayberry (Myrica rubra) populations and identification of sex-associated markers. BMC Genomics 16, 394 (2015).
Chai, C. Y. et al. Studies on characteristics of androphyte inflorescence and pollination of Myrica rubra. J. Zhejiang Forestry Sci. Technol. 31, 1–5 (2011).
Wang, G. et al. Construction of a crossing population between two Chinese bayberry cultivars ‘Biqi’ and ‘Dongkui’ and hybrid identification by polymorphic SSRs. J. Fruit. Sci. 32, 555–560 (2015).
Yu, Q. et al. Recent origin of dioecious and gynodioecious Y chromosomes in papaya. Tropical Plant Biol. 1, 49–57 (2008).
Zhang, Q. et al. High-density interspecific genetic maps of kiwifruit and the identification of sex-specific markers. DNA Res. 22, 367–375 (2015).
Akagi, T. & Charlesworth, D. Pleiotropic effects of sex-determining genes in the evolution of dioecy in two plant species. Proc. R. Soc. B 286, 20191805 (2019).
Akagi, T. et al. Two Y-chromosome-encoded genes determine sex in kiwifruit. Nat. Plants 5, 801–809 (2019).
Westergaard, M. The mechanism of sex determination in dioecious plants. Adv. Genet. 9, 217–281 (1958).
Brautigam, K. et al. Sexual epigenetics: gender-specific methylation of a gene in the sex-determining region of Populus balsamifera. Sci. Rep. 7, 45388 (2017).
Aranzana, M. J. et al. Prunus genetics and applications after de novo genome sequencing: achievements and prospects. Hortic. Res. 6, 58 (2019).
Zhao, H. B. et al. Genome-wide identification and analysis of the MADS-box gene family and its potential role in fruit development and ripening in red bayberry (Morella rubra). Gene 717, 144045 (2019).
Davis, T. M. et al. An examination of targeted gene neighborhoods in strawberry. BMC Plant Biol. 10, 81–81 (2010).
Xu, C. et al. Identifying the genetic diversity, genetic structure and a core collection of Ziziphus jujuba Mill. var. jujuba accessions using microsatellite markers. Sci. Rep. 6, 31503 (2016).
Erfani, J. et al. Genetic diversity of some pear cultivars and genotypes using simple sequence repeat (SSR) markers. Plant Mol. Biol. Rep. 30, 1065–1072 (2012).
Hou, X. J. et al. Genome-wide identification, classification, expression profiling, and SSR marker development of the MADS-box gene family in citrus. Plant Mol. Biol. Rep. 32, 28–41 (2014).
Hollister, J. D. et al. Indel-associated mutation rate varies with mating system in flowering plants. Mol. Biol. Evol. 27, 409 (2010).
Zhang, S. M. et al. Genetic diversity of Chinese bayberry (Myrica rubra Sieb. et Zucc.) accessions revealed by amplified fragment length polymorphism. Hortscience 44, 487–491 (2009).
Terakawa, M. et al. Characterization of 13 polymorphic microsatellite loci for an evergreen tree, Myrica rubra. Mol. Ecol. Notes 6, 709–711 (2006).
Jia, H. M. et al. Development of 107 SSR markers from whole genome shotgun sequences of Chinese bayberry (Myrica rubra) and their application in seedling identification. J. Zhejiang Univ. Sci. B 15, 997–1005 (2014).
Zhang, S. M. et al. Development and characterization of microsatellite markers for Chinese bayberry (Myrica rubra Sieb. & Zucc.). Conserv. Genet. 10, 1605–1607 (2009).
Van Ooijen, J. W. JoinMap® 4.1, Software for the calculation of genetic linkage maps in experimental populations of diploid species. (Kyazma B.V., Wageningen, 2012).
Stokes, J. Cytological studies in the Myricaceae. Botanical Gaz. 99, 387–399 (1937).
Kosambi, D. D. The estimation of map distance from recombination values. Ann. Hum. Genet. 12, 172–175 (1943).
Voorrips, R. E. MapChart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 93, 77–78 (2002).
Pritchard, J. K. et al. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Earl, D. A. & Vonholdt, B. M. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361 (2012).
Acknowledgements
We would like to thank Dr. Eric van de Weg for his valuable suggestions about linkage map construction. Z.S.G. acknowledges support from the Natural Science Foundation of China (31972364) and Yuyao Forestry Technology Extension Center. H.M.J. was supported by the National Natural Science Foundation of China (31901985). Z.S.G., Y.J., C.Y.C. were funded by a Ningbo Science and Technology Research Grant (contract no 2016C10021).
Author information
Authors and Affiliations
Contributions
Z.S.G., H.M.J., and H.J.J. initiated this project. G.Y.W., H.J.J., Y.J., C.C.Z., L.J.Y., D.L.S., and C.Y.C. performed crossing, seedling cultivation and sample collection. Y.T.S., Y.W., H.M.J., X.W.L., C.Q.Z., and H.B.Z. tested the SSR markers and performed genotyping. Y.W., Q.S.Y., and H.M.J. constructed the linkage map. Y.W., Q.S.Y., and Z.S.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Jia, HM., Shen, YT. et al. Construction of an anchoring SSR marker genetic linkage map and detection of a sex-linked region in two dioecious populations of red bayberry. Hortic Res 7, 53 (2020). https://doi.org/10.1038/s41438-020-0276-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41438-020-0276-6
This article is cited by
-
SSR Marker Acquisition and Application from Transcriptome of Captive Chinese Forest Musk Deer (Moschus berezovskii)
Biochemical Genetics (2023)
-
Assessment of the genetic variability and population structure in boro rice cultivars of Assam, India using candidate gene based SSR markers
Genetic Resources and Crop Evolution (2023)
-
Comprehensive analysis of SSRs and database construction using all complete gene-coding sequences in major horticultural and representative plants
Horticulture Research (2021)
-
The de novo genome assembly of Tapiscia sinensis and the transcriptomic and developmental bases of androdioecy
Horticulture Research (2020)