Abstract
Many studies suggest that there are distinct regulatory processes controlling compound leaf development in different clades of legumes. Loss of function of the LEAFY (LFY) orthologs results in a reduction of leaf complexity to different degrees in inverted repeat-lacking clade (IRLC) and non-IRLC species. To further understand the role of LFY orthologs and the molecular mechanism in compound leaf development in non-IRLC plants, we studied leaf development in unifoliate leaf (un) mutant, a classical mutant of mungbean (Vigna radiata L.), which showed a complete conversion of compound leaves into simple leaves. Our analysis revealed that UN encoded the mungbean LFY ortholog (VrLFY) and played a significant role in leaf development. In situ RNA hybridization results showed that STM-like KNOXI genes were expressed in compound leaf primordia in mungbean. Furthermore, increased leaflet number in heptafoliate leaflets1 (hel1) mutants was demonstrated to depend on the function of VrLFY and KNOXI genes in mungbean. Our results suggested that HEL1 is a key factor coordinating distinct processes in the control of compound leaf development in mungbean and its related non-IRLC legumes.
Similar content being viewed by others
Introduction
Plant leaves are the primary photosynthetic organs that are initiated on the flanks of the shoot apical meristem (SAM). The class I KNOTTED1-like homeobox (KNOXI) genes are involved in the maintenance of the meristem activity of SAM, while the initiation of leaves requires downregulation of KNOXI genes at the incipient site1,2,3. In simple-leafed species such as Arabidopsis thaliana, downregulation of KNOXI genes in leaf primordia is permanent, whereas in most compound-leafed eudicot species, including the tomato (Solanum lycopersicum) and Cardamine hirsuta, KNOXI genes are reactivated in leaf primordia after initiation of leaf development4,5,6. In C. hirsuta, the leaflet number is reduced in mutants of the KNOXI gene SHOOTMERISTEMLESS (ChSTM) or BREVIPEDICELLUS (ChBP)7,8. In S. lycopersicum, ectopic expression of the KNOXI genes Tomato KNOTTED1 (Tkn1) or Tkn2 (orthologs of STM and BP in tomato, respectively) in transgenic lines, or upregulated expression of Tkn1 or Tkn2 in related mutants, results in ramification for compound leaves suggesting that regulatory processes mediated by KNOXI genes, especially STM/BP-like KNOXI genes, play pivotal roles in compound leaf development5,9,10.
However, in the inverted repeat-lacking clade (IRLC) of legumes, which includes Pisum sativum and Medicago truncatula, the expression of STM/BP-like KNOXI genes is excluded from leaf primordia11,12,13. Genetic analysis shows that single mutants, double mutants and triple mutants of 3 STM/BP-like KNOXI genes, namely, MtKNOX1, MtKNOX2, and MtKNOX6, in M. truncatula do not show obvious defects in compound leaves13. Thus, STM/BP-like KNOXI genes may not be involved in compound leaf development in IRLC legume plants11,12,13. Instead, another type of transcription factor, UNIFOLIATA (UNI) in P. sativum and SINGLE LEAFLET1 (SGL1) in M. truncatula, orthologs of LEAFY (LFY) from Arabidopsis, functions in controlling compound leaf development14,15,16. The uni mutants in pea and sgl1 mutants in M. truncatula exhibit single leaflet phenotypes, and inflorescence and floral defects15,16. Hence, the LFY orthologs appear to play a significant role in compound leaf development in IRLC legumes. Furthermore, it has been shown that the UNI cofactor UNUSUAL FLORAL ORGANS (UFO) in pea, and PALM1, an upstream transcription factor of SGL1 in M. truncatula, are involved in the control of leaf complexity17,18. Recent studies show that the adaxial–abaxial regulators PHANTASTICA (PHAN), ARGONAUTE7 (AGO7), and AUXIN RESPONSIVE FACTOR 3 (ARF3) regulate the expression level of PALM1 and therefore control compound leaf development in M. truncatula19,20,21.
The function of the LFY orthologs during compound leaf development has also been investigated in non-IRLC legumes, including soybean and L. japonicus in which KNOXI proteins are expressed in leaves, and are likely associated with compound leaf development12,22. In L. japonicus, a mutant of the LFY ortholog Proliferating Floral Meristem (PFM) exhibits one or two reduced basal leaflets12. In soybean LFY-RNAi-silenced lines, only the leaflet number of the compound leaves produced at the second node is reduced12. This would indicate that there is a minor role in compound leaf development for LFY orthologs in non-IRLC legume species12,22,23.
In this study, we described the compound leaf developmental processes in a non-IRLC legume species, mungbean (Vigna radiata L.), a fast-growing (60–90 days) warm-season grain legume, and characterized the unifoliate leaf (un) mutants that showed a complete conversion of compound leaves into simple leaves. Four alleles of un carried mutations in the LFY ortholog, indicating that the LFY ortholog in mungbean played a significant, rather than a minor role in compound leaf development. Phylogenetic analysis of the KNOX gene family in legumes was conducted, and the expression of four STM/BP-like KNOXI genes was characterized in mungbean using in situ RNA hybridization. Furthermore, genetic interaction and gene expression analysis showed that increased leaflet number in heptafoliate leaflets1 (hel1) mutants involved regulatory processes mediated by VrLFY and STM/BP-like KNOXI genes in mungbean. This study showed that the LFY ortholog might play a more significant role in the control of compound leaf development earlier than the time estimated by Champagne et al.12.
Materials and methods
Plant material and growth conditions
All the mutants were isolated from the M2 population of a mutagenized mungbean cultivar, Sulu, generated in Nanjing, China. The gamma irradiator was calibrated to irradiate the seed lots with 400 Gy of gamma rays. The M1 seeds were sown in the field, and the M2 seeds were individually harvested from the population. Approximately 36 seeds of each M2 line were planted in individual rows in the field, with a distance of 0.3 m between rows. The mutant plants were then individually harvested and sown for further observation in the greenhouse at 26–30 °C with a 16-h light/8-h dark photoperiod at 200 μmol m−2 s−1. The allelic nature of genes was confirmed by crosses among un1-1, un1-2, un1-3, and un1-4, using heterozygote parents because the mutants were sterile (the mutant plants were found in F1 plants of the crosses). The L. japonicus ecotype Gifu B-129 was grown at 20–22 °C with a 16-h light/8-h dark photoperiod at 150 μmol m−2 s−1 in the greenhouse.
Scanning electron microscopy
Mungbean shoot apices at different developmental stages were fixed in FAA solution containing 3.7% (v/v) formaldehyde, 50% (v/v) ethanol, and 5% (v/v) acetic acid. Before vacuum freeze drying, fixed samples were dehydrated in an ethanol/tert-butanol series. The materials were transferred to a vacuum freeze dryer overnight. The preparation of shoot apices for scanning electron microscopy (SEM) analysis was as described by Chen et al.24. Samples were examined in a JEOL JSM-6360LV (JEOL) SEM at 10–15 kV of acceleration voltage.
Molecular cloning of the full-length LFY gene (VrLFY) from mungbean
To clone the full-length mungbean LFY gene, we first searched the genome sequence database of mungbean (http://plantgenomics.snu.ac.kr) using sequences for UNI and SGL1. Finally, sequence alignment with UNI/SGL1/LFY/FLO coding sequences allowed the open reading frame of VrLFY to be defined. Polymerase chain reaction (PCR) was carried out using the primers in Supplementary Table 3. PCR products were cloned into the pGEM-T easy vector (Promega), and inserts were characterized by nucleotide sequencing.
In situ hybridization
For in situ probes, gene-specific regions of VrLFY, VrKNOXI, and LjKNOXI genes were generated by PCR with primer sets (see Supplemental Table 4) and cloned into a pGEM-T vector (Promega, A1360). In situ probes were synthesized from these clones by in vitro transcription using the Digoxigenin RNA Labeling Kit (Roche) from either the T7 or SP6 promoter flanking the insert, generating either sense or anti-sense probes. Mungbean shoot apices were fixed overnight in 4 % (wt/vol) paraformaldehyde, pH 7.0, and then embedded in Paraplast (Sigma). RNase-free slices of the shoot apices were hybridized to digoxigenin-labeled probes and used for subsequent immunological detection as previously described25.
Expression analysis by quantitative reverse transcription PCR
Shoot apices from 2-week-old mutant and wild-type plants were collected in RNase-free tubes and stored in a −80 °C freezer. Samples were taken in triplicate as biological replicates. Total RNA was extracted using the Plant RNA Kit (Omega) following the manufacturer’s instructions. Samples were then treated with RNase-free DNase I (Promega) for 30 min.
For quantitative reverse transcription PCR (qRT-PCR), first-strand cDNA was synthesized from total RNA using the First Strand cDNA Synthesis Kit (Fermentas). Real-time RT-PCR analysis was performed as three technical replicates in 384-well plates using SYBR® Premix ExTaq™ (Takara), on an ABI StepOnePlus machine, according to the manufacturer’s protocol (Applied Biosystems). The relative expression level of genes was determined by the 2−ΔΔCT method. Amplification of VrTUB (Vradi05g13910), a constitutively expressed gene, was used as an internal control to normalize all data26. Shoot apices from a single genotype were represented by nine samples; independent total RNA isolations were generated from the three biological replicates, and three technical qRT-PCR replicates were performed on each of the total RNA preparations. The primers used for qRT-PCR are given in Supplementary Table 5.
Transcript profiling by deep-sequencing
For Illumina sequencing, mRNA was purified from shoot apices of 2-week-old seedlings of un1-1 mutant and wild-type plants and then fragmented into small pieces. Random hexamer primers and reverse transcriptase (Invitrogen) were used to carry out first-strand cDNA synthesis. Second-strand cDNA synthesis was performed with DNA polymerase I (New England BioLabs) after RNase H (Invitrogen) treatment. Four cDNA libraries were constructed, and cDNA sequencing was conducted using the Illumina HiSeq X Ten system according to the manufacturer’s protocol, with read lengths of 150 bp. The raw data were submitted to the NCBI Short Read Archive (SRP110723). The number of reads per kilobase of exon region in a gene per million mapped reads (RPKM) was used to normalize the gene expression data27,28. Differentially expressed genes were determined between the wild type and mutants according to statistical analysis of the frequency of each transcript, and their corresponding P-values were calculated25. The significance threshold of P-values in multiple tests was set by the false discovery rate (FDR). We used a FDR ≤ 0.05 and an absolute value of |log2 ratio| ≥ 1 as the threshold to judge the significance of gene expression differences.
Phylogenetic analysis
The phylogenetic tree was constructed using the Molecular Evolutionary Genetics Analysis software (MEGA; version 6.0) by the neighbor-joining method (JFF Matrix model) with 1000 bootstrap replications.
Data availability
Sequence data from this article can be found in the GenBank data libraries under the following accession numbers: XP_014491863.1 (VrLFY); XP_017441945.1 (VaLFY); XP_007137848.1 (PhvLFY); XP_003526918.2 (GmLFY1); XP_014630701.1 (GmLFY2); AAX13294.1 (PFM); AAC49782.1 (UNI); XP_003602745.1 (SGL1); XP_002284664.1 (VFL); AF197934_1 (FALSIFLORA); AAM27941.1 (LEAFY); AAA62574.1 (FLORICAULA); AQQ16908.1 (ChLFY); XP_015635355.1 (RFL); and O04407.1 (NEEDLY).
Results
Compound leaf development in mungbean
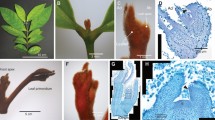
Similar to M. truncatula, L. japonicus, and other compound leaf-producing species, leaf development in mungbean was heteroblastic. The first pair of juvenile leaves with simple leaf morphologies emerged in opposite phyllotaxy on the first node of a developing mungbean plant and was succeeded by adult trifoliate leaves in alternate phyllotaxy (Fig. 1a–c). The wild-type trifoliate leaves of mungbean consisted of a pair of stipules, a petiole, 2 lateral leaflets, a rachis, and a single terminal leaflet (Fig. 1c). To facilitate the characterization of leaf mutants in mungbean, we investigated leaf developmental processes by scanning electron microscopy (SEM). The morphological changes during compound leaf development in mungbean can be divided into seven distinct stages. At Stage 0 (S0), cells along the periphery of SAM were recruited as founder cells and became an incipient leaf primordium (Fig. 1d). At S1, a common leaf primordium formed as a strip of cells grew out along the periphery of SAM (Fig. 1d). At the subsequent S2, a pair of stipule primordia emerged at the proximal end of the common leaf primordium (Fig. 1e), enlarged, and then separated away from the common leaf primordium so that boundaries were established between the two stipules and the common leaf primordium at S3 (Fig. 1f). At S4, a pair of lateral leaflet primordia emerged between the stipule and the common leaf primordium; and then the common leaf primordium differentiated into a terminal leaflet primordium (Fig. 1g). Subsequently, at S5, the lateral leaflet primordia were separated away from a terminal leaflet primordium so that a boundary was established, and trichomes initiated from the abaxial surface of the terminal leaflet primordium (Fig. 1h). Following S5, the lateral leaflets and terminal leaflet primordium became folded (Fig. 1i), and the region between the stipule and lateral leaflet primordia expanded to become a petiole as a result of cell division and cell expansion at the S6 stage.
a Whole plant morphology of mungbean. The arrow indicates the opposite juvenile leaves at the first node. b A pair of juvenile leaves of mungbean. c Adult compound leaf of mungbean. d–i SEM analysis of compound leaf development. d Sites of the incipient leaf primordia were specified at the periphery of the SAM at S0. At S1, a common leaf primordium was initiated as a strip of cells outgrowing along the periphery of SAM. e A pair of stipule primordia (ST) was initiated from the proximal end of the common leaf primordium at S2. f At S3, the boundaries (arrows) between the stipule and lateral leaflet primordia were formed. g At S4, a pair of lateral leaflet primordia (LL) emerged between the stipule and common leaf primordium. h At S5, the common leaf primordium differentiated into a terminal leaflet primordium (TL) as indicated by development of trichomes from the abaxial surface. Boundaries (arrows) were formed between the lateral and terminal leaflet primordia. i At S6, the leaflet primordia folded as a result of outgrowth of the abaxial surface, and the region between the stipule and lateral leaflet primordia expanded to form a petiole (P). Trichomes developed from the abaxial surface of both the stipule and lateral leaflet primordia. b, c Scale bars = 1 cm; d–i Scale bars = 50 μm
Isolation and characterization of un mutants in mungbean
Four similar leaf mutants (Supplementary Table 1), which mimicked the phenotype of the classical mutant un, were isolated from a mutant population of mungbean generated by gamma irradiation29. Genetic analyses demonstrated that they were allelic (Materials and Methods section). In these mutants, all adult leaves consisted of a short petiole bearing a single terminal leaflet (Fig. 2a, b). Compared with the terminal leaflet of the wild type, the single terminal leaflet of the mungbean un mutant was larger (Fig. 2b). We found that the heteroblastic progression was also delayed in un mutants such that two simple leaves were produced at the first and second nodes in opposite phyllotaxis. Flowers that developed in un mutants were abnormal and infertile. un mutants produced sepal-like proliferating structures that lacked petals and stamens, and the number of whorls of organs within the flowers was increased (Fig. 2c). Because of their infertility, un mutants were maintained as heterozygotes. Progeny from self-pollination of heterozygous lines segregated in a 3:1 ratio (33 wild-type plants and 9 mutant plants, χ2 = 0.28 < χ20.05 = 5.99), suggesting that the mutant phenotype was controlled by a single-recessive gene.
a Wild-type mungbean (left) and un1-1 mutant (right) exhibiting compound and simple leaf forms, respectively. Arrows indicate the opposite juvenile leaves at the first node in the wild type and at the first and second nodes in the un1-1 mutant. b Close-up views of the adult leaves of the wild type (left) and un1-1 mutant (right). c Morphology of a mature un1-1 mutant (right), exhibiting simple leaf and floral homeotic phenotypes compared to the wild type (left). The inset has a close-up view of the inflorescence of the un1-1 mutant. d, f Leaf development of wild type at S4 and S6. e, h Leaf development of un1-1 at S4 and S6. The lateral leaflet primordia did not form at the proximal end of the common leaf primordium. LL lateral leaflet, TL terminal leaflet, SL single leaflet. b–c Scale bars = 10 cm; d–f 50 μm indicated
To characterize leaf development defects in mungbean un mutants, SEM analysis of leaf development was conducted. This analysis indicated that in un mutants, leaf development was initially normal until S4, at which point, the pair of lateral leaflet primordia failed to initiate between the stipule and the common leaf primordium (Fig. 2d, e). All four alleles of the mungbean un mutants exhibited this identical defect. The defect in the initiation of the lateral leaflet primordia was persistent throughout subsequent developmental stages, resulting in the formation of simple adult leaves in the un mutants of mungbean (Fig. 2f, g).
Molecular cloning of VrLFY in mungbean
The floral homeotic defects and single-leaf phenotype of the un mutants resembled that of the uni mutant in pea and the sgl1 mutant in M. truncatula15,16. The full-length DNA sequence of the LFY ortholog (VrLFY) in mungbean was obtained from the mungbean genomic database; the genomic sequence of VrLFY was 2155 bp in length. Alignment of the genomic sequence of VrLFY with its cDNA sequence showed the existence of two introns (Supplementary Figure 1a). PCR amplification of mungbean genomic LFY from the un mutants and from the wild-type plants indicated that three un alleles (un1-1, un1-2, and un1-3) carried deletions (Fig. 3a). Nucleotide sequencing showed that another allele, the un1-4 mutant, had only a single base-pair substitution from the wild type gene (GAA to GGA, Supplementary Figure 1a). This resulted in an amino acid change (E112G, where the acidic amino acid Glu was replaced by a neutral amino acid Gly) in the N terminal domain of the protein (Supplementary Figure 1b).
a PCR amplification of the VrLFY gene from wild-type mungbean and un mutants (WT, un1-1, un1-2, un1-3, and un1-4). Deletions were detected as no product on attempted amplification of the VrLFY gene from three mutant alleles. b Phylogenetic analysis of VrLFY and its putative orthologs: VaLFY of V. angularis, PvLFY of Phaseolus vulgaris, GmLFY1 and GmLFY2 of soybean, PFM of L. japonicas, UNI of pea, SGL1 of M. truncatula, VFL of Vitis vinifera, FALSIFLORA of tomato, FLORICAULA of snapdragon, LEAFY of Arabidopsis, ChLFY of C. hirsute, RFL of rice and NEEDLY of Pinus radiata. Bootstrap supports above 50 % from 1000 replicates are shown. c, d VrLFY gene expression was detected in SAM and developing leaf primordia. e The VrLFY sense probes were used as a negative control, and no hybridization signal was detected in SAM and leaf primordia. Scale bars = 100 μm
Segregation analysis of an F2 population of the un1-1 allele indicated that 50 out of a total of 208 individuals were homozygous for the deletion and exhibited both simple leaf and floral homeotic defects, suggesting that the deletion in the corresponding VrLFY gene cosegregated with the mutant phenotype. Thus, the locus of un in mungbean was allelic to VrLFY, which encoded a putative plant-specific transcription factor closely related to UNI in pea and SGL1 in M. truncatula (Fig. 3b). In situ RNA hybridization data revealed that the VrLFY gene was expressed in the SAM and in the emerging leaf primordia (Fig. 3c) and showed relatively high expression in the distal portion of leaf primordia (Fig. 3d).
Characterization and expression analysis of STM/BP-like KNOXI genes in mungbean
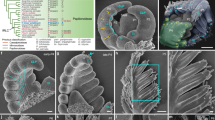
To characterize the KNOXI genes in mungbean, we conducted a BLAST search of its genome (http://plantgenomics.snu.ac.kr), verifying candidate genes with the public transcriptome dataset30. Sixteen KNOX proteins were identified from mungbean and these were divided into three classes (class I, class II, and class M) based on phylogenetic analysis (Supplementary Figure 2)30. Nine proteins were classified as STM-like KNOXI proteins (Vradi07g26830, Vradi10g07810 and Vradi06g03570), BP-like KNOXI protein (Vradi06g14320) and KNAT2/6-like KNOXI proteins (Vradi08g11380, Vradi03g07470, Vradi05g04350, Vradi11g09640, and Vradi0322S00070). Five KNOXII proteins were classified as KNAT3/4/5-like proteins (Vradi05g1039, Vradi05g03240, and Vradi07g21010) and KNAT7-like proteins (Vradi11g02470 and Vradi07g13210). In addition, there were two members of class M KNOX proteins found in our mungbean sequence search (Vradi01g05360 and Vradi11g11780).
It has been reported that STM/BP-like KNOXI genes in tomato and C. hirsuta are expressed in the compound leaf and are involved in the control of lateral leaflet development4,5,6. However, STM/BP-like KNOXI genes are not associated with compound leaf development in M. truncatula and pea11,12,13. Previously, accumulation of KNOXI proteins in compound leaf primordia of non-IRLC legumes was detected by polyclonal KNOXI-specific antibodies12. To compare the expression patterns of STM/BP-like KNOXI genes in mungbean with those of IRLC-legume and model plant species, in situ RNA hybridization of four STM/BP-like KNOXI genes (Vradi07g26830, Vradi10g07810, Vradi06g03570, and Vradi06g14320) was carried out on sections of apices from 2-week-old mungbean seedlings (Fig. 4). The results showed that there were different expression patterns among the four STM/BP-like genes in mungbean (Fig. 4a–d). The expression of the two STM-like genes Vradi10g07810 and Vradi06g03570 was strongly detected in the shoot apical meristem, and transcripts were also observed in the leaf primordia (Fig. 4b, c). However, expression of the STM-like gene Vradi07g26830 and the BP-like gene Vradi06g14320 was detected in the SAM but not in the leaf primordia (Fig. 4a, d).
Therefore, our data showed that the expression patterns of 2 STM-like KNOXI genes from mungbean differed from pea and M. truncatula, as in those species, no STM-like genes have been shown to be expressed in any stage of the compound leaf primordia13. Moreover, BP-like genes were not expressed in the compound leaf primordia of mungbean, L. japonicus, pea and M. truncatula, which is a different situation from the expression of these genes in C. hirsuta and tomato4,5,7,11,13,22.
Transcript profiling of VrLFY downstream targets
To address the molecular function of VrLFY during leaf development, the transcriptome of shoot apices of 2-week-old seedlings from un1-1 mutant and wild-type plants was studied using RNA-Seq. A total of 538 differentially expressed genes (300 downregulated and 238 upregulated) were identified between the mutant and wild-type plants (Supplementary Table 2). The results revealed a significant representation of genes associated with circadian rhythm and plant hormone signal transduction (Supplementary Table 2). In the un1 mutants, the genes encoding proteins with high similarity to GIGANTEA 3 (GI3), GIGANTEA-like, Phytochrome A (PHYA), TIME OF CAB EXPRESSION 1 (TOC1), CIRCADIAN CLOCK ASSOCIATED 1 (CCA1), EARLY FLOWERING 3 (ELF3), and Adagio protein 3 (ADO3) were significantly downregulated, while those with high similarity to LATE ELONGATED HYPOCOTYL (LHY) were significantly upregulated. It has been reported that these are key factors in the regulation of the circadian clock and regulate important developmental transitions such as flowering, which was consistent with the involvement of LFY and its orthologs in controlling flowering time and phase transition31,32,33.
Previous studies have reported that plant hormones, including auxin and gibberellins (GA), play critical roles in leaflet initiation and compound leaf development34,35. In our expression studies, auxin, GA, ethylene and cytokinin-related genes were significantly differentially expressed in mutants compared to wild-type plants (Supplementary Table 2). In addition, a number of receptor-like protein kinases were differentially expressed, which might imply that several signaling cascades involved in cell proliferation and differentiation play a significant role in the control of compound leaf development in mungbean.
The transcripts of the KNOX family genes showed no obvious differential expression (data not shown), with the exception that one of the class M KNOX genes, Vradi11g11780, was decreased by approximately eightfold in vegetative shoot apices of the un1-1 mutant compared with the wild-type plant (Supplementary Table 2), and this was confirmed by qRT-PCR (data not shown). It has been reported that increasing the expression of class M KNOX genes in Arabidopsis and tomato results in serrated leaves and a larger number of leaflets, respectively36,37.
Genetic interactions affecting compound leaf development in mungbean mutants
Other mutants that showed an increase in the number of leaflets were identified, including heptafoliate leaflets1 (hel1) and small-pentafoliate leaflets1 (smp1), with 2 and 3 alleles, respectively (Fig. 5a–c; Supplementary Table 1). The juvenile leaves of the hel1 mutant showed an extreme dissection and leaflet-like structures, sometimes associated with stipules, which developed in the proximal part of the blade (Supplementary Figure 3). The juvenile leaves of the smp1 mutant were normal, but the adult leaves exhibited five leaflets of small size (Fig. 5c). We made crosses between hel1 and smp1 to examine genetic interactions. In the resulting F2 population, there were four classes of leaflet size and number: trifoliate, heptafoliate, small-heptafoliate, and small-pentafoliate. The numbers of plants in the different leaflet classes approximated a 9:3:3:1 ratio of the respective phenotypes (data not shown), suggesting that there were 2 unlinked genes controlling the multiple leaflet trait in mungbean. Further genetic analysis showed that the small-heptafoliate plants were hel1 smp1 double mutants (Fig. 5d), indicating that there was no additive phenotype of the double mutation in terms of leaflet number and that HEL1 interacted genetically with SMP1 in the control of leaflet number in mungbean compound leaf development.
Compound leaf phenotype of the wild type and mutants. a–f Mature compound leaves of a WT, b hel1-1, c smp1-1, d hel1-1 smp1-1, e un1-1, and f hel1-1 un1-1 mutants (all in the Sulu ecotype). Leaves of the hel1-1 un1-1 double mutants exhibited three leaflets with short petioles. The hel1-1 smp1-1 double mutants were heptafoliate leaves of small size, indicating an epistatic interaction between hel1 and smp1 in the control of leaflet number. Scale bars = 10 cm
To genetically test the involvement of un in the proliferation of lateral leaflet primordia in the hel1 mutant, we generated hel1-1 un1-1 double mutants. Juvenile plants of the double mutant were similar to that of a hel1 single mutant. However, all adult leaves in the hel1-1 un1-1 double mutants consisted of a short petiole bearing two lateral leaflets and one terminal leaflet (Fig. 5f), indicating the requirement for the VrLFY gene in the proliferation of lateral leaflet primordia in the hel1 mutant. In addition to the alterations in leaflet number, the hel1 and un mutants also exhibited alterations in the proximal-distal axis of compound leaves. Compared with wild-type leaves, the petiole length of mature leaves in the hel1 mutant was increased by approximately 10%, while the mature leaves of the un mutant exhibited short petioles (Fig. 5b, e). Interestingly, the petiole length of mature leaves of hel1-1 un1-1 double mutants was short (Fig. 5f), resembling those of the un single mutant, suggesting that un is genetically epistatic to hel1 in leaf petiole development.
HEL1 regulates the expression of VrLFY and STM/BP-like KNOXI genes
The genetic analysis described above indicated that HEL1 controls trifoliate development via 2 distinct pathways, either dependent or independent of LFY. We first examined the expression of VrLFY and its putative upstream transcription factor PALM1 (VrPALM1) and cofactor UFO (VrUFO) in the wild type and hel1 mutants17,18. qRT-PCR data revealed that in vegetative shoot apices of the hel1 mutant compared with wild-type plants, VrLFY and VrUFO transcript levels were increased by threefold and fourfold, respectively (Fig. 6a). However, VrPALM1 transcript levels showed no obvious change in the hel1 mutant compared with wild-type plants. Because some KNOXI genes expressed in the compound leaf were likely associated with compound leaf development in mungbean, we further examined the expression of KNOX genes in the hel1 mutant and wild-type plants. The results showed that the expression of the 4 STM/BP-like KNOXI genes was significantly upregulated (Fig. 6b), while other KNOXI genes were not upregulated (data not shown). Interestingly, a KNOXM gene, Vradi11g11780 (Supplementary Figure 2), was also upregulated by fourfold in the hel1 mutant (Fig. 6a). Taken together, these data suggest that HEL1 regulates the expression of LFY and KNOXI genes to determine the leaflet number in mungbean.
qRT-PCR analysis of gene expression relative to that of the mungbean TUB gene. The level of transcripts was examined in the shoot apices of mutants compared with wild-type plants 2 weeks after seed germination. Bars represent means ± SEs (n = 3). Vradi07g26830 is a KNOXM gene. Vradi07g26830, Vradi10g07810, Vradi06g03570, and Vradi06g14320 are STM/BP-like KNOXI genes
Discussion
The role of the LFY orthologs in compound leaf development in legumes
In most compound-leafed species, activation of KNOXI gene expression in the leaf primordia is correlated with the development of compound leaves6,12. However, in IRLC legume species with compound leaves such as pea and M. truncatula, the STM/BP-like KNOXI genes are excluded from the leaf primordia, and their expression is not correlated with compound leaf development11,12,13. In these plants, the LFY orthologs appear to function in place of the KNOXI genes in controlling compound leaf development12,15,16. The pea uni mutants and M. truncatula sgl1 mutants exhibit compound leaf defects, with all adult leaves changed to simple leaves, indicating that the LFY orthologs play a significant role in compound leaf development in IRLC legumes15,16.
The available information on the role of LFY orthologs in compound leaf development in non-IRLC legumes comes from phenotypic analysis of L. japonicus pfm mutants and soybean LFY transgenic lines12,22. In the pfm mutants, compound leaves lack 1 or 2 basal leaflets22. In transgenic soybean lines in which the endogenous LFY genes are downregulated, only the leaflet number of the compound leaves produced at the second node is reduced12. In tomato, the mutant of the LFY ortholog, fa, has a reduced number of small leaflets present on the compound leaf32. Recently, it has been reported that the lfy mutant in C. hirsuta shows a lower number of leaflets than the wild type33. Therefore, it seems that the single leaflet phenotype caused by mutations of the LFY orthologs is only exhibited in IRLC legumes. It is thought that the LFY orthologs acquired a more significant role no earlier than the divergence of the Hologalegina clade from the other legumes12.
However, our results showed that the un mutants in mungbean, with a complete conversion of compound leaves into simple leaves, were loss-of-function mutations of VrLFY. This finding indicated that VrLFY could play a significant rather than a minor role in compound leaf development in mungbean, a member of the non-IRLC legumes (Fig. 2a–c). Interestingly, mutants exhibiting simple leaves and malformed flowers have also been reported in other non-IRLC legumes, including adzuki bean and cowpea38,39. While unproven, one could speculate that some of these mutant phenotypes in adzuki bean and cowpea, with similarities to the un mutants in mungbean, could also be caused by mutations of LFY orthologs. If so, LFY orthologs could play a significant role in compound leaf development in other non-IRLC legumes, not just in mungbean. In addition, the question of when in evolution LFY orthologs acquired a significant role in compound leaf development might need to be re-addressed12. Further investigation of the function of LFY orthologs in leaf development of different clades of non-IRLC legumes would be helpful in answering this question.
Expression pattern of STM/BP-like KNOXI genes in legumes
Phylogenetic analysis showed that there were duplications of KNOXI genes in legumes (Supplementary Figure 2). There was 1 STM gene and 1 BP gene in Arabidopsis, tomato and C. hirsuta. However, in IRLC legumes such as M. truncatula and pea, there were 2 STM-like genes and 1 BP-like KNOXI gene. In non-IRLC legumes such as mungbean and L. japonicus, there were 3 STM-like genes and 1 BP-like KNOXI gene. In situ RNA hybridization showed that the 2 STM-like KNOXI genes in mungbean were expressed at the compound leaf primordia (Fig. 4), which was different from that of pea and M. truncatula in which none of the STM-like genes were expressed in any stage of the compound leaf primordia11,13. BP-like genes were not expressed in the compound leaf primordia of mungbean (Fig. 4) and L. japonicus or in pea and M. truncatula5,7,9,10,11,13. This result was in contrast to findings of BP orthologs from C. hirsuta and tomato, which were expressed in the compound leaf primordia25. It has been shown that differences in expression patterns between BP from Arabidopsis and ChBP from C. hirsuta are attributable to their cis-regulatory regions7. When KNOXI genes are overexpressed in M. truncatula and Alfalfa, there is an increase in leaflet number12,13. The loss of the role for KNOXI genes in compound leaf development of IRLC legumes also likely occurred due to a loss of expression12,13. However, this loss of expression of the STM-like and BP-like genes in compound leaf primordia in IRLC legumes could have occurred at different times in evolution because BP-like genes were also not expressed in compound leaf primordia in non-IRLC legumes, such as mungbean and L. japonicus.
VrLFY could interact with KNOXI in mungbean to regulate compound leaf development
In simple-leafed species, LFY orthologs play a key role in phase transition and floral development. Many downstream targets and DNA binding motifs of LFY have been identified at the genomic level in the model plant Arabidopsis40,41. Despite its key role in compound leaf development in species such as pea and M. truncatula, how the LFY orthologs regulate downstream genes to affect lateral organ development, especially that of compound leaf development, remains elusive. In this study, transcriptomic analysis uncovered a total of 538 differentially expressed genes between mutants and the wild type. Several types of key factors, such as CCA1 and ELF3, involved in flowering time and phase transition were among the significantly differentially expressed genes (Supplementary Table 2), which was consistent with the conserved function of LFY orthologs in plants.
LFY has been reported to regulate the expression of some KNOXI genes such as BP and KNAT2 in Arabidopsis during pedicel and flower development41,42. However, the transcription levels of the KNOXI genes showed no obvious change in the un mutant compared with the wild-type plant (data not shown), suggesting that VrLFY does not regulate the expression of KNOXI genes at the transcriptional level in mungbean. Nevertheless, 1 class M KNOX gene, Vradi11g11780, with high similarity to PETROSELIUM (PTS) in tomato and FUSED COMPOUND LEAF (FCL2) in M. truncatula, was downregulated 3.5-fold in the un1-1 mutant37,43. It has been shown that the class M KNOX proteins in Arabidopsis and tomato could form heterodimers with BEL1-like homeodomain (BELL) proteins and interfere with the regulatory networks of KNOXI-BELL complexes during leaf development36. Transgenic Arabidopsis lines overexpressing KNATM-B exhibit serrated leaves, and a mutant with upregulated expression of the PTS gene in tomato exhibits a proliferation of compound leaves36,37. In M. truncatula, the class M KNOX gene FCL1 has been shown to control boundary establishment and petiole length of compound leaves and is required for the development of extra leaflet primordia in a palm1 mutant44. Therefore, our results indicated that VrLFY might modulate KNOXI regulatory networks by regulating the expression of a class M KNOX gene (Vradi11g11780) to control compound leaf development. Mutant databases for model legume plants such as M. truncatula and L. japonicus and legume crops such as soybean are available45,46,47. It would be worth identifying mutants of Vradi11g11780/FCL2 orthologs and dissecting their roles in compound leaf development in different legumes. Furthermore, it will also be necessary to identify mutations of the STM/BP-like KNOXI genes in mungbean and other non-IRLC legumes to investigate gene and protein interactions between KNOXI genes and the LFY orthologs during compound leaf development in non-IRLC plants.
HEL1 could orchestrate VrLFY and KNOXI to control compound leaf development in mungbean
Our results suggested that there were 2 distinct regulatory processes mediated by the LFY ortholog and KNOXI proteins during compound leaf development in mungbean. It also raised the question of how the two processes were coordinated during compound leaf development in mungbean. The HEL1 gene was a key locus of mungbean in the control of leaflet number, whose mutation resulted in dissected juvenile leaves and heptafoliate adult leaves (Fig. 5b and Supplementary Figure 3). Double mutant analysis showed that hel1 genetically interacted with un and smp1 to control the leaflet number of the compound leaves, indicating that lateral leaflet formation in the hel1 mutant was dependent not only on LFY but also on other regulators in the control of compound leaf development. Consistently, gene expression analysis showed that the STM/BP-like KNOXI genes and a KNOXM gene, VrLFY, as well as its putative cofactor VrUFO, were significantly upregulated in the hel1 mutant compared to the wild type (Fig. 6). Therefore, these results suggested that HEL1 could coordinate the regulatory processes mediated by VrLFY and KNOXI to control compound leaf development in mungbean.
Interestingly, heptafoliate-leaf-like mutants similar to the hel1 mutant in mungbean were also identified and characterized in other legumes, including soybean and cowpea48,49. In soybean, the seven-leaflet character is a single recessive trait conditioned by the lf2 locus45. Preliminary mapping results in mungbean revealed that the HEL1 gene was located to a region of chromosome 11, which showed synteny with the lf2 locus in soybean (data not shown)50. Future work to clone the HEL1 gene and its ortholog in soybean and related legumes could provide new insights into the molecular mechanisms orchestrating the two regulatory processes mediated by the LFY ortholog and KNOXI genes during compound leaf development in mungbean and other legumes.
References
Long, J. A., Moan, E. I., Medford, J. I. & Barton, M. K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69 (1996).
Sinha, N. R., Williams, R. E. & Hake, S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes Dev. 7, 787–795 (1993).
Jackson, D., Veit, B. & Hake, S. Expression of maize KNOTTED 1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120, 405–413 (1994).
Hareven, D., Gutfinger, T., Parnis, A., Eshed, Y. & Lifschitz, E. The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell 84, 735–744 (1996).
Chen, J. J., Janssen, B. J., Williams, A. & Sinha, N. A gene fusion at a homeobox locus: alterations in leaf shape and implications for morphological evolution. Plant Cell 9, 1289–1304 (1997).
Bharathan, G. et al. Homologies in leaf form inferred from KNOXI gene expression during development. Science 296, 1858–1860 (2002).
Hay, A. & Tsiantis, M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat. Genet. 38, 942–947 (2006).
Rast-Somssich, M. I. et al. Alternate wiring of a KNOXI genetic network underlies differences in leaf development of A. thaliana and C. hirsuta. Genes Dev. 29, 2391–2404 (2015).
Parnis, A. et al. The dominant developmental mutants of tomato, Mouse-ear and Curl, are associated with distinct modes of abnormal transcriptional regulation of a Knotted gene. Plant Cell 9, 2143–2158 (1997).
Shani, E. et al. Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell 21, 3078–3092 (2009).
Hofer, J., Gourlay, C., Michael, A. & Ellis, T. H. Expression of a class 1 knotted1-like homeobox gene is down-regulated in pea compound leaf primordia. Plant Mol. Biol. 45, 387–398 (2001).
Champagne, C. E. et al. Compound leaf development and evolution in the legumes. Plant Cell 19, 3369–3378 (2007).
Zhou, C. et al. STM/BP-like KNOXI is uncoupled from ARP in the regulation of compound leaf development in Medicago truncatula. Plant Cell 26, 1464–1479 (2014).
Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859 (1992).
Hofer, J. et al. UNIFOLIATA regulates leaf and flower morphogenesis in pea. Curr. Biol. 7, 581–587 (1997).
Wang, H. et al. Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula. Plant Physiol. 146, 1759–1772 (2008).
Taylor, S., Hofer, J. & Murfet, I. Stamina pistilloida, the Pea ortholog of Fim and UFO, is required for normal development of flowers, inflorescences, and leaves. Plant Cell 13, 31–46 (2001).
Chen, J. et al. Control of dissected leaf morphology by a Cys(2)His(2) zinc finger transcription factor in the model legume Medicago truncatula. Proc. Natl Acad. Sci. USA 107, 10754–10759 (2010).
Ge, L. & Chen, R. (2014). PHANTASTICA regulates leaf polarity and petiole identity in Medicago truncatula. Plant Signal. Behav. 9, e28121 (2017).
Zhou, C. et al. The transacting short interfering RNA3 pathway and no apical meristem antagonistically regulate leaf margin development and lateral organ separation, as revealed by analysis of an argonaute7/lobed leaflet1 mutant in Medicago truncatula. Plant Cell 25, 4845–4862 (2013).
Peng, J., Berbel, A., Madueño, F. & Chen, R. AUXIN RESPONSE FACTOR3 regulates compound leaf patterning by directly repressing PALMATE-LIKE PENTAFOLIATA1 expression in Medicago truncatula. Front. Plant Sci. 8, 1630 (2017).
Wang, Z. et al. Multiple components are integrated to determine leaf complexity in Lotus japonicus. J. Integr. Plant Biol. 55, 419–433 (2013).
Wang, Y. & Chen, R. Regulation of compound leaf development. Plants 3, 1–17 (2013).
Chen, C., Wang, S. & Huang, H. LEUNIG has multiple functions in gynoecium development in Arabidopsis. Genesis 26, 42–54 (2002).
Luo, J. H. et al. Different expression patterns of duplicated PHANTASTICA-like genes in Lotus japonicus suggest their divergent functions during compound leaf development. Cell Res. 15, 665–677 (2005).
Sairam, R. K., Dharmar, K., Chinnusamy, V. & Meena, R. C. Water logging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J. Plant. Physiol. 166, 602–616 (2009).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Audic, S. & Claverie, J. M. The significance of digital gene expression profiles. Genome Res. 7, 986–995 (1997).
Sangsiri, C., Sorajjapinun, W. & Srinivesc, P. Gamma radiation induced mutations in mungbean. ScienceAsia 31, 251–255 (2005).
Kang, Y. J. et al. Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 5, 443–5451 (2014).
Shim, J. S., Kubota, A. & Imaizumi, T. Circadian clock and photoperiodic flowering in Arabidopsis, CONSTANS is a hub for signal integration. Plant Physiol. 173, 5–15 (2017).
Molinero-Rosales, N. et al. FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem identity. Plant J. 20, 685–693 (1999).
Monniaux, M. et al. Conservation vs divergence in LEAFY and APETALA1 functions between Arabidopsis thaliana and Cardamine hirsuta. New Phytol. 216, 549–561 (2017).
Hay, A. et al. The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr. Biol. 12, 1557–1565 (2002).
Barkoulas, M., Hay, A., Kougioumoutzi, E. & Tsiantis, M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 40, 1136–1141 (2008).
Magnani, E. & Hake, S. KNOX lost the OX, The Arabidopsis KNATM gene defines a novel class of KNOX transcriptional regulators missing the homeodomain. Plant Cell 20, 875–887 (2008).
Kimura, S., Koenig, D., Kang, J., Yoong, F. Y. & Sinha, N. Natural variation in leaf morphology results from mutation of a novel KNOX gene. Curr. Biol. 18, 672–677 (2008).
Blakeslee, A. F. A unifoliolate mutation in adzuki bean. J. Hered. 4, 153–155 (1919).
Rawal, K. M., Porter, W. M., Franckowiak, J. D., Fawole, I. & Rachie, K. O. Unifoliolate leaf, A mutant in cowpeas. J. Hered. 67, 193–194 (1976).
Moyroud, E. et al. Prediction of regulatory interactions from genome sequences using a biophysical model for the Arabidopsis LEAFY transcription factor. Plant Cell 23, 1293–1306 (2011).
Winter, C. M. et al. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Dev. Cell. 20, 430–443 (2011).
Yamaguchi, N., Yamaguchi, A., Abe, M., Wagner, D. & Komeda, Y. LEAFY controls Arabidopsis pedicel length and orientation by affecting adaxial-abaxial cell fate. Plant J. 69, 844–856 (2012).
Di Giacomo, E. et al. KNAT3/4/5-like class 2 KNOX transcription factors are involved in Medicago truncatula symbiotic nodule organ development. New Phytol. 213, 822–837 (2017).
Peng, J. et al. Regulation of compound leaf development in Medicago truncatula by fused compoundleaf1, a class M KNOX gene. Plant Cell 23, 3929–3943 (2011).
Tadege, M. et al. Large scale insertional mutagenesis using Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J. 54, 335–347 (2008).
Małolepszy, A. et al. The LORE1 insertion mutant resource. Plant J. 88, 306–317 (2016).
Li, Z. et al. Development and utilization of a new chemically-induced soybean library with a high mutation density. J. Integr. Plant Biol. 59, 60–74 (2017).
Fehr, W. R. Genetic control of leaflet number in soybeans. Crop Sci. 12, 221–224 (1972).
Fawole, I. Genetic control of leaflet number and shape in cowpea, Vigna unguiculata (L) Walp. Niger. J. Sci. 34, 73–80 (2000).
Seversike, T. M., Ray, J. D., Shultz, J. L. & Purcell, L. C. Soybean molecular linkage group B1 corresponds to classical linkage group 16 based on map location of the lf (2) gene. Theor. Appl. Genet. 117, 143–147 (2008).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31700186) and the Ministry of Agriculture of China for Transgenic Research (Grant No. 2014ZX0800943B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
41438_2018_88_MOESM2_ESM.jpg
Supplementary Figure 2. Phylogenetic analysis of members of KNOX gene family in mungbean, L. japonicus, M. truncatula, pea, and Arabidopsis
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiao, K., Li, X., Su, S. et al. Genetic control of compound leaf development in the mungbean (Vigna radiata L.). Hortic Res 6, 23 (2019). https://doi.org/10.1038/s41438-018-0088-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41438-018-0088-0
This article is cited by
-
An effective transient expression system for gene function identification in Lotus japonicus
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Control of compound leaf patterning by MULTI-PINNATE LEAF1 (MPL1) in chickpea
Nature Communications (2023)
-
Fabaceae leaf morphogenetic evolution: the leaf-lamina architectural variation in the Fabaceae flora of Indian Western Ghats, compared with that genetically characterized in the Fabaceae model species Pisum sativum and Medicago truncatula
Proceedings of the Indian National Science Academy (2021)
-
A molecular framework underlying the compound leaf pattern of Medicago truncatula
Nature Plants (2020)
-
LOVE ON WINGS, a Dof family protein regulates floral vasculature in Vigna radiata
BMC Plant Biology (2019)