Abstract
Sexually antagonistic coevolution is predicted to lead to the divergence of male and female genotypes related to the effects of substances transferred by males at mating on female physiology. The outcome of mating should thus depend on the specific combination of mating genotypes. Although mating has been shown to influence female immunity in diverse insect taxa, a male–female genotype-by-genotype effect on female immunity post mating remains largely unexplored. Here, we investigate the effects of mating on female decorated cricket baseline immunity and the potential for a male-genotype-by-female-genotype interaction affecting this response. Females from three distinct genotypic backgrounds were left unmated or singly mated in a fully reciprocal design to males from the same three genotypic backgrounds. Hemocytes and hemocyte microaggregations were quantified for female cellular immunity, and phenoloxidase, involved in melanization, and antibacterial activity for humoral immunity. In this system, female cellular immunity was more reactive to mating, and mating effects were genotype-dependent. Specifically, for hemocytes, a genotype-by-mating status interaction mediated the effect of mating per se, and a significant male–female genotype-by-genotype interaction determined hemocyte depletion post mating. Microaggregations were influenced by the female’s genotype or that of her mate. Female humoral immune measures were unaffected, indicating that the propensity for post-mating effects on females is dependent on the component of baseline immunity. The genotype-by-genotype effect on hemocytes supports a role of sexual conflict in post-mating immune suppression, suggesting divergence of male genotypes with respect to modification of female post-mating immunity, and divergence of female genotypes in resistance to these effects.
Similar content being viewed by others
Introduction
Sexual conflict ensues when individuals pursue reproductive strategies that are detrimental to the fitness of their mates (Chapman et al. 2003). Such conflicts can occur before, during, or after mating, and can manifest in various ways. An especially pervasive conflict arises over female remating rates in polyandrous species (Arnqvist and Nilsson 2000; Chapman et al. 2003; Rowe and Arnqvist 2002). Although females can increase their reproductive success by trading up in the quality of their mates, rematings with additional males invariably increase the likelihood of sperm competition, resulting in decreased paternity of females’ respective mating partners (Parker and Birkhead 2013). In an attempt to mitigate these costs, males may evolve reproductive tactics that function to control female remating (Arnqvist and Rowe 2002; Dougherty et al. 2017). In various insect species, for example, males attempt to thwart female remating using various behavioral means, including prolonged copulation, passive-phase guarding in which males continue to physically grasp females after mating, and, more commonly, through mate guarding (Alcock 1994; Arnqvist 1988; Cordero 1990, 1999; Sakaluk 1991; Sherman 1983).
In addition to controlling female remating rates using behavioral tactics, males of some species attempt to control female remating via substances transferred during mating. In several insect orders, a portion of the male’s ejaculate serves as a mating plug that functions to prevent subsequent insemination by other males (Baer et al. 2001; Dickinson and Rutowski 1989; Lung and Wolfner 2001). More broadly, the seminal fluids of various male insects contain seminal fluid proteins (accessory gland proteins) that may also influence female remating through their effects on female physiology and behavior (Gillott 2003; Klowden 1999; Sakaluk et al. 2006). In Drosophila melanogaster, for example, seminal proteins have been identified that reduce female receptivity to further courtship attempts by other males (Wigby and Chapman 2005; Wolfner 1997). Insect seminal fluid proteins are also known to manipulate other facets of female reproduction post mating (reviewed in Avila et al. 2010). Work in Drosophila has elegantly connected certain seminal proteins with mating-induced changes in female egg production and ovulation, as well as changes in female immunity (reviewed in Ravi Ram and Wolfner 2007). Sex peptide is a widely investigated seminal fluid protein in Drosophila that is known to carry out several functions, including inducing upregulation of antimicrobial peptides (AMPs) in mated females. Peng et al. (2005) demonstrated that the expression of the AMPs, metchnikowin, drosomycin, and diptericin, is stimulated shortly after mating in female Drosophila. Alteration of female immunity post mating through physiological effects of male-derived substances is one of multiple ways that mating can influence female immunity (Lawniczak et al. 2007). The corruption or enhancement of female immunity by mating and its subsequent influence on infection outcomes will affect female lifetime fitness and the evolutionary landscape of sexual conflict.
Manipulation of female physiology and particularly female immunity to the advantage of the mating male is central to the immunogenic male hypothesis (Morrow, Innocenti 2012). Such effects of mating on female immunity may be pleiotropic, stemming from changes to other aspects of female physiology, or could result from direct selection for a benefit to males. The immunogenic male hypothesis focuses on post-mating activation of female immunity, such as immune gene expression in Drosophila (Peng et al. 2005) and resistance to infection in Gryllus texensis (Shoemaker et al. 2006). However, if female immune suppression post mating leads to the reallocation of limited resources to immediate reproduction, and hence increased paternity of the current male, it could also result in sexual conflict (Fedorka et al. 2007; Lawniczak et al. 2007; Short and Lazzaro 2010).
It is predicted that sexual conflict followed by sexually antagonistic coevolution should lead to the rapid divergence of male and female genotypes related to the effects of seminal proteins or other male-derived substances on female physiology (Goenaga et al. 2015; Haerty et al. 2007; Rice and Holland 1997). As a consequence of divergence driven by sexually antagonistic coevolution, males from different populations and genotypes should differ in the manipulative effect of transferred substances, and similarly, females should differ in their susceptibility to these compounds. The outcome of mating for female physiology can therefore be expected to depend on male and female genotypes, but ultimately the combination of the two, which results in a genotype-by-genotype interaction. It has been demonstrated that the expression of female Drosophila immune genes post mating depends on the interaction between male and female genotypes (Delbare et al. 2017). However, such genotype-by-genotype interactions on female immune gene expression were not uncovered in a recent study of reciprocal crosses of two D. melanogaster populations (Fricke et al. 2020). Furthermore, another study investigating realized immunity on infection in Drosophila found suppression of female post-mating immunity to be pathogen dependent and genetic variation across female genotypes in post-mating immune suppression, but no interaction between male and female genotypes in crosses between nine male and nine female genotypes (Short and Lazzaro 2010). Taken together, these results suggest that in Drosophila there is limited evidence for the hypothesis that sexual conflict universally drives changes to female post-mating immunity, despite the occurrence of genotype-by-genotype interactions in some circumstances (Delbare et al. 2017). However, the effects of male and female genotypes on female post-mating immunity have not been explicitly investigated in other species where sexual conflict is also predicted to be prevalent.
The decorated cricket (Gryllodes sigillatus) has become a focal study organism for investigating sexual conflict over female mating behavior (Sakaluk et al. 2019), primarily because of the nuptial food gifts offered by males to females at mating that are known to influence female physiology and behavior (Gershman et al. 2012; 2013; Sakaluk 2000; Sakaluk et al. 2006; Warwick et al. 2009). The spermatophore transferred by a male during copulation comprises a small sperm-containing ampulla enveloped by a gelatinous spermatophylax that the female detaches from the ampulla and consumes immediately after mating as a form of nuptial feeding. Once the female has finished consuming the spermatophylax, she also detaches and eats the sperm ampulla, terminating sperm transfer (Sakaluk 1984; 1987). Free amino acids contained within the spermatophylax function to enhance its gustatory appeal, extending the time the female spends feeding on it before she discards it (Gershman et al. 2012; 2013; Warwick et al. 2009), thereby promoting greater sperm transfer and enhancing male fertilization success (Sakaluk 1986; Sakaluk and Eggert 1996; Eggert et al. 2003). In addition, the food gifts of male G. sigillatus contain proteins that may serve to manipulate female sexual receptivity and physiology (Sakaluk et al. 2006). Indeed, a recent proteomic analysis has identified a number of proteins in the spermatophylax with the potential to manipulate components of female reproductive physiology (Pauchet et al. 2015). Therefore, in addition to seminal proteins transferred at mating, as in Drosophila (Ravi Ram and Wolfner 2007), the spermatophylax may act as a conduit for the effects on the post-mating immunity of female G. sigillatus.
Previous studies have revealed that mating can influence immunity in decorated crickets, specifically with respect to trade-offs between reproductive effort and immunity in males (Gershman et al. 2010b; Kerr et al. 2010). For example, Gershman et al. (2010b) identified a phenotypic trade-off between lytic activity, an important facet of antibacterial immunity, and the mass of the spermatophylax synthesized by the male. Kerr et al. (2010) demonstrated a reciprocal trade-off between immunity and reproduction in G. sigillatus, in which immune-challenged males produced smaller spermatophores, whereas experimentally induced spermatophore production in males resulted in decreased immune function. More recently, Duffield et al. (2018) showed that the circulating hemocyte numbers of male crickets are increased at 4 h after a bacterially based immune challenge, and that such an immune challenge can influence male reproductive effort in a context-dependent manner. A previous study identified differences in immunity among males and females of different G. sigillatus inbred lines (Gershman et al. 2010a). However, the effects of mating on female immunity in G. sigillatus are still largely unknown.
Here, we investigate how mating per se affects female immunity in decorated crickets, the influence of female genotype on these effects, and also whether male genotype interacts with female genotype to influence female immunity post mating. To assess the effects of mating on female baseline potential immunity, we compared the measures of female cellular and humoral immunity between mated and virgin females within three distinct genotypic backgrounds from established inbred lines (Ivy et al. 2005), in the absence of activation by an infective agent. To determine whether male genotype interacts with female genotype in mediating differences in female immunity post mating, we assigned females of these known genotypes to mate with males from the same or a different genotypic background, after which female cellular and humoral immune parameters were assessed. Based on previous studies investigating the effects of mating on immunity in insects (Lawniczak et al. 2007; Schwenke et al. 2016), we predicted that mated females would differ from virgin females in one or more parameters of immunity. However, if genetic variation in modulation of female immunity in decorated crickets is maintained by ongoing sexually antagonistic coevolution, resulting from sexual conflict, we predicted that the extent of post-mating changes to female immune profiles would be affected by the specific combination of the mating male and female genotypes.
Materials and methods
Study animals
G. sigillatus used in this study were randomly selected from three genetically distinct inbred lines (designated E, F, and I) that were established in 2001 from a wild-caught population of approximately 500 individuals collected in Las Cruces, New Mexico. Inbred lines were created by subjecting crickets to 23 generations of full-sib mating, followed by panmixia within lines thereafter (Ivy et al. 2005). Previous work has revealed that these inbred lines vary in a number of phenotypic and life-history traits, including differences in lifespan, female fecundity, male-calling effort, nuptial gift composition, and immune function (Archer et al. 2012; Duffield et al. 2019; Gershman et al. 2010a, b; 2013). Lines have been demonstrated to differ in phenoloxidase activity, encapsulation, and microaggregation formation, but not humoral antibacterial activity (Duffield et al. 2019; Gershman et al. 2010a). The lines used in this study were all matching in terms of the stage of development at the beginning of the experiment, enabling matings of age-controlled individuals between lines. Due to the logistics of carrying out specific and controlled matings between lines, but still maintaining the desired age control, the samples were collected across four generations. Samples from all genotypes were collected from each generation. Given that each inbred line is considered genetically homogeneous, there should be limited variation between individuals across generations. However, generation was added into initial models of all analyses to investigate and account for any differences resulting from variation across generations.
All crickets were maintained under standard rearing conditions and lines, and individuals were kept under identical environments. Individuals were housed in 19 l ventilated, plastic storage bins lined with egg carton to increase rearing surface area and provided with cat chow (Purina Cat Chow CompleteTM), rodent meal (Envigo© 2018CM Teklad Certified Global 18% protein rodent diet), and water (in glass vials plugged with cotton) ad libitum. To ensure their virginity, juvenile males and females were separated when sex differences became apparent (4th or 5th instar). Females were individually housed in clear 0.47 l PET plastic deli containers, whereas males were housed in groups of roughly ten males per line in 5.68 l ventilated, plastic boxes. All individuals were housed in an environmental chamber at 32 °C on a 14-h:10-h light:dark cycle. Juvenile males and females were checked twice weekly for eclosion. At the time of mating, all experimental females were 7–9 day-old adults and males were 4–12 day-old adults. Female G. sigillatus becomes sexually mature 2–4 days post adult eclosion and male G. sigillatus 4–11 days post adult eclosion (Burpee and Sakaluk 1993; Sakaluk 1987).
Mating observations
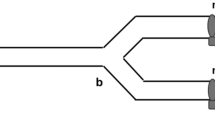
Sexually mature females were randomly assigned to a mating status treatment: mated (singly) or virgin. Genotype-by-genotype treatments were nested within the mated treatment, with females randomly paired with a male from their same line or from one of the other two inbred lines, creating a fully reciprocal design of all possible male and female genotype combinations (Fig. 1). To confirm mating success, crickets were viewed under red light in clear plastic viewing chambers (10.5 × 7.5 × 3 cm) lined with paper towels (Ivy and Sakaluk 2005). All mating trials took place during the dark period of their light cycle to capture a time most relevant to their mating behavior in nature (Sakaluk 1987; Sakaluk et al. 2002). For each mating pair, courtship and copulation behaviors were recorded from the time the male was introduced into the viewing chamber. These included time to courtship (i.e., comprising both song and distinctive vibratory movements by the male to entice the female to mount), time to female mounting (i.e., a female’s decision to accept a mate), time to mating (i.e., successful transfer of the spermatophore from the male to the female), time to gift consumption (i.e., when the female removed the spermatophylax from the spermatophore), time to termination of gift consumption (i.e., when the female finished eating the gift, either fully consuming it or prematurely discarding it), and time to removal of the sperm ampulla (i.e., when the female terminates sperm transfer by removing the sperm ampulla). Because courtship is essential for mating to occur in crickets (Sakaluk 1987), if a male did not initiate courtship within the first 10 min of being introduced into the mating chamber, he was removed from the chamber and replaced with a different male from the same line. Females, on average, were provided with 2.4 different males over the course of 2 days until mating took place. If a female did not mate within 2 days, she was removed from the experiment. For females that did mate but did not remove the sperm ampulla themselves after finishing nuptial gift consumption, the sperm ampulla was manually removed after 50 min, which is enough time to ensure complete sperm transfer (Sakaluk 1984). For all mated females, the total number of males she was provided, her age, and the age of her mate was recorded. To control for any potential differences resulting from exposure to a male conspecific per se (Zhong et al. 2013), females assigned to the virgin mating status treatment were placed with a juvenile male for 60 min. While this is the best control available, it should be noted that it may only represent a partial control in the likely event that, independent of mating, sexually mature and juvenile males interact with females differently.
Numbers within cells represent sample sizes. Each replicate sample represents a unique female that was, when mated, paired with a unique male. Due to logistical constraints, samples were collected across four generations. Females (i.e., replicates) across lines and treatments were balanced across generations and within generations with respect to time, thereby avoiding any possible confound between treatment and time. *Due to sample loss, the number samples of virgin females for lines E and F were reduced by one for antibacterial activity.
Quantifying female immunity
Immunity of mated females was assessed 24 h after mating or at the corresponding time point for virgin females. This time frame is similar to other studies in insects that have demonstrated mating effects on female immunity (Barribeau and Schmid-Hempel 2017; Fedorka et al. 2004; Peng et al. 2005; Rolff and Siva-Jothy 2002). To quantify immune function, four measures were carried out that encompass both cellular and humoral aspects of insect immunity (Gillespie et al. 1997). Specifically, we measured (i) counts of the total number of circulating hemocytes, (ii) presence of hemocyte microaggregations representing cellular immunity, (iii) enzymatic activity of total phenoloxidase (PO), and (iv) cell-free antibacterial activity representing humoral immunity. Because insect immunity is multifaceted, measuring components of both cellular and humoral immunity captures a suite of potential female immune responses to mating (Gillespie et al. 1997; Shoemaker et al. 2006).
Hemolymph was extracted from cold-anesthetized females by piercing the membrane above the dorsal pronotum plate with a sterile 25-G needle. Four microliters of outflowing hemolymph were collected with a chilled microcapillary tube at the puncture site. The collected hemolymph was expelled and mixed into 11 μl of chilled Grace’s Insect Medium (MilliporeSigma, CAS: G8142). This first dilution was to be used for the antibacterial activity assay. Four microliters of this first dilution were taken and added to a further 20 μl of chilled Grace’s Insect Medium for assaying the enzymatic activity of total PO. An additional 4 μl of the first dilution was added to 15 μl of chilled Grace’s Insect Medium to quantify circulating hemocytes and the presence of microaggregations. Circulating hemocytes and microaggregations were immediately counted following hemolymph collection from samples kept on ice, whereas samples for antibacterial and total PO activity were snap-frozen in liquid nitrogen and stored at –80 °C for later analysis. Body size, measured as pronotum width, was taken as a covariate for all females.
Antibacterial activity assay
Antibacterial activity of cell-free hemolymph is an important component of insect humoral immunity. This antibacterial activity includes the action of both lysozyme-like enzymes and AMPs. Some highly conserved AMPs, such as defensin (Yi et al. 2014), exhibit a broad range of antibacterial activity, targeting both Gram-positive and Gram-negative bacteria (Gillespie et al. 1997). Insect lysozymes also defend against Gram-positive bacteria through the catalyzation of hydrolysis of glycosidic bonds in bacterial cell wall peptidoglycan (Schneider 1985).
A zone of inhibition assay, following established protocols for this species (Duffield et al. 2018), was used to assay the humoral antibacterial activity of all experimental females. Diluted hemolymph samples were added to petri dishes containing agar seeded with the Gram-positive Micrococcus luteus (ATCC 4698). Micrococcus luteus used in the assay was taken from a single colony on a pregrown streak plate, added to 7 ml of liquid media (2 g of peptone and 1.2 g of meat extract in 400 ml of nanopure water, pH 7), and grown in liquid culture for 48 h at 30 °C. Following quantification of cell number, a fraction of this culture was added to liquid media containing 1% agar (i.e., seeded medium) held at 40 °C to achieve a final density of 1.5 × 105 cells/ml. Six milliliters of seeded medium were poured evenly into a 100-mm-diameter petri dish and allowed to solidify. Sample wells were made in the solidified seeded medium using a Pasteur pipette (Volac D810). Hemolymph samples were thawed on ice and 2.5 μl of samples were added to individual wells. Measurements of antibacterial activity were replicated by adding hemolymph samples from each female to two separate petri dishes. A negative control of Grace’s Insect Medium was also included on each plate. After adding samples to wells, plates were inverted and incubated for 48 h at 30 °C, after which standardized images were taken of each sample well under a dissecting microscope, and the diameter of clear inhibition zones was measured. Images with a millimeter scale were used for calibration, and for each sample, two measurements of zone diameter were taken perpendicular to one another using ImageJ (Schneider et al. 2012). These measures of one technical replicate were averaged, and measured zone diameters were converted to units of lysozyme (mg/ml), based on a standard curve of the zone of inhibition measurements from hen egg white lysozyme (MilliporeSigma, CAS: 12650-88-3). For each individual, the average activity in lysozyme units of the technical replicates was used in subsequent analyses. Zones were measured blind to treatment.
Total phenoloxidase activity assay
The phenoloxidase, or melanization, cascade is another important part of the humoral response of insects. At the onset of this cascade, a serine protease cleaves prophenoloxidase (proPO), the inactive zymogen form of PO that exists in the hemolymph, to create the active form of PO. After activation, PO catalyzes the production of melanin, as well as phenols, quinones, and other cytotoxins (Nappi and Vass 1993; Sugumaran et al. 2000) to defend against multicellular pathogens and parasites, bacteria, fungi, and viruses (González-Santoyo and Córdoba-Aguilar 2012; Soderhall and Cerenius 1998; Sugumaran et al. 2000).
To measure total PO activity, samples were thawed on ice and 10 μl of each diluted sample were added to an individual well of a 96-well microplate (CytoOne) preprepared with 135 μl of chilled nanopore water, 20 μl of phosphate buffer solution (pH 6.5), and 5 μl of chemotrypsin (5 mg/ml, MilliporeSigma, CAS: C4129), and then allowed to incubate at 20 °C for 15 min. During this incubation period, chemotrypsin cleaves PO from proPO, simulating the natural activation step (Soderhall and Cerenius 1998). After incubation, the plate was returned to ice, and 20 μl of L-DOPA (4 mg/ml, MilliporeSigma, CAS: D9628), the reactant in the melanization cascade that is converted to dopaquinone by PO, was added to each well. Dopaquinone spontaneously converts to dopachrome causing a colormetric change that was measured by recording optical density (OD) of the solution with a spectrophotometer (Thermo Scientific Multiskan GO) at 490 nm. OD readings were taken every 20 s for 60 min with the plate incubated at 30 °C. The maximal rate of enzymatic activity (Vmax) is measured as the slope of the reaction curve (change in OD/time) during its linear phase and is expressed as ΔOD/h. Samples were run blind to treatment, in duplicate and averaged for each individual.
Circulating hemocyte counts and microaggregations
Hemocytes are specialized cells key to insect cellular immunity, being involved in coagulation, phagocytosis, and encapsulation (Lavine and Strand 2002). In addition, hemocytes are known to phagocytose microorganisms and subsequently form small aggregates (i.e., microaggregations) during the early stages of nodule formation in an attempt to clear large numbers of microbes from circulation (Gillespie et al. 1997). Immediately after extraction, hemolymph was added to a counting chamber (Fast-Read® 102, Immune Systems Ltd., UK) and viewed at ×400 magnification under a phase-contrast microscope. Hemocyte counts and the presence of microaggregations were recorded for each individual. Counting was performed blind to treatment and, for consistency, by the same person for all samples. Depending on the density of hemocytes, variable numbers of counting chamber grids were viewed for quantification. Accounting for the number of viewed grids and the dilution, hemocyte counts were converted to counts per microliter of hemolymph. The number of grids was also used as an offset in analyses of the presence of microaggregations observed during the counting of hemocytes.
Statistical analysis
All analyses were performed in R version 3.6.3 “Holding the Windsock” for Mac (R Core Team 2019). The survival package (Therneau 2020) was used for Cox proportional-hazard models and the MASS and lme4 packages for generalized linear models (Bates et al. 2015). For each response variable, potential distributions were assessed for model fit and adherence to model assumptions. Initial models were simplified by sequentially eliminating nonsignificant terms through F tests or likelihood ratio tests (LRTs) using the function drop1, and nested models were compared and selected using AIC (Burnham and Anderson 2002). Statistics for terms not in the final models were taken from the step before their removal. The package emmeans (Lenth et al. 2020) was used to calculate estimated marginal means and their confidence intervals for levels of model terms and for pairwise comparisons of factor levels or combinations of factor levels in interactions with Tukey HSD used for multiplicity adjustment.
Mating behavior
Using data from all successful matings, Cox proportional-hazard regression models were used to evaluate the effect of male genotype, female genotype, their interaction, generation, and the covariate of body size on (1) time to female mounting, indicating mate acceptance, (2) time spent feeding on the nuptial gift (spermatophylax), and (3) time from the attachment to the removal of the sperm ampulla. For the analysis of ampulla-attachment time, nuptial gift consumption time was also included as a covariate, as it is known from previous studies that time spent feeding on the gift influences the timing of ampulla removal (Sakaluk 1984). The exact option was specified in the Cox proportional-hazard model statement to handle ties, instances in which different females had the same time for any of the aforementioned mating parameters, because this option assumes that mating events are continuous and ordered, assumptions that are likely met by our data. There was no censoring in the cases of time to female mounting and termination of female gift consumption, as these events occurred in all cases. However, for sperm ampulla-attachment time, females were included as right-censored observations when the sperm ampulla was manually removed after 50 min (i.e., after complete sperm transfer had occurred) if the female had terminated gift consumption but not removed the ampulla herself.
The influence of female genotype and mating status on immunity in the context of within-line crosses
Comparing virgin and mated females, the effect of mating per se on the measures of female immunity was analyzed in within-line crosses. Only within-line crosses were used in this analysis to avoid confounding any effect of mating with an effect of male genotype. Initial models included body size as a covariate, generation, female genotype, mating status, and the interaction between female genotype and mating status. Humoral immune measures of total PO and antibacterial activity were analyzed with general linear models. The response variable of antibacterial activity was log-transformed to meet model assumptions, and this approach was preferred as it produced a better fitting model than generalized linear model distributions with log-link functions. Due to overdispersion of count data, the number of circulating hemocytes per microliter of hemolymph and microaggregation counts were analyzed with generalized linear models with negative binomial distributions and log-link functions. Although 56% of the female microaggregation counts were zero, this was predicted accurately by the fitted negative binomial distribution. However, to account for differential sampling effort in recording the presence of microaggregation, the number of counting chamber grids observed was included as an offset in the model (Hardin and Hilbe 2007).
The influence of male genotype, female genotype, and their interaction on post-mating female immunity
Data from all mated females were used to assess the effect of male genotype, female genotype, their interaction, generation, and the covariate of female body size on post-mating female immunity. The responses of total PO activity, cell-free antibacterial activity of the hemolymph, the number of circulating hemocytes per microliter of hemolymph, and microaggregation counts were analyzed using models set up in the same way as the models investigating mating status and female genotype described above. Any significant male-genotype-by-female-genotype interactions were further investigated by categorizing male–female pairings as either “self” for within-line crosses or “other” for between-line crosses, and subsequently used this coded variable to analyze if differences in the pairing type underlie any significant differences.
Results
Mating behavior
Mating females took on average 535 s (s.d. 420 s) to mount males. There was no significant effect of generation (LRT X23 = 0.845, p = 0.8387), female body size (LRT X21 < 0.001, p = 0.9916), male genotype (LRT X22 = 0.168, p = 0.9196), or the interaction between male and female genotype (LRT X24 = 1.326, p = 0.8569) on time to mount. However, there was a significant effect on time to mounting of female genotype (LRT X22 = 6.031, p = 0.049, Supplementary Fig. 1). Females of line E took longer to mount (Hazard 0.791 [95% C.I. 0.643–0.972]) than line-F females (1.226 [1–1.504]), with line I females intermediate (1.026 [0.844–1.247]). The mean time females spent feeding on the nuptial gift was 1635 s (s.d. 910 s), with no significant effect of generation (LRT X23 = 3.246, p = 0.3553), female body size (LRT X21 < 0.001, p = 0.9864), male genotype (LRT X22 = 0.808, p = 0.6676), or the interaction between male and female genotype (LRT X24 = 3.024, p = 0.5538). Mirroring the time to mount, there was, however, a significant effect of female genotype on the time females spent feeding on the spermatophylax (LRT X22 = 10.703, p = 0.0047, Supplementary Fig. 2), with consumption time being longer in line E females (Hazard 0.685 [95% C.I. 0.543–0.865]) than females of both lines F (1.185 [0.948–1.482]) and I (1.214 [0.972–1.516]). Although sperm ampulla attachment times differed between female lines, this was driven by an expected significant positive effect of spermatophylax attachment time (LRT X21 = 48.232, p < 0.0001), and when this was included in the model, there was no significant effect on ampulla attachment time of generation (LRT X23 = 6.590, p = 0.0867), female size (LRT X21 = 0.033, p = 0.8564), female genotype (LRT X22 = 2.537, p = 0.2812), male genotype (LRT X22 = 1.004, p = 0.6052), or the interaction between male and female genotypes (LRT X24 = 3.501, p = 0.4777).
Female immunity
The influence of female genotype and mating status on immunity in the context of within-line crosses
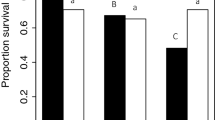
There was a significant effect of female body size on humoral antibacterial activity, with larger females having greater activity (β = 0.208, Table 1). However, there was no significant difference in antibacterial activity between virgin females (estimated marginal mean in mg/ml lysozyme [95% C.I.] = 1.63 [1.37–1.93]) and mated females (1.99 mg/l [1.68–2.36]) (Table 1). There was also no significant effect of generation, female genotype, or the interaction between female genotype and mating status (Table 1 and Fig. 2A).
A Virgin and mated females from three genotypic backgrounds (E, F, and I). For this analysis, all matings were within genotypic backgrounds. B Females from the three genotypic backgrounds (E, F, and I) mated to male genotypes (E, F, and I) to produce all possible own and other line-mating combinations.
For total phenoloxidase activity, mated females (estimated marginal mean ΔOD/h [95% C.I.] = 0.69 [0.64–0.75]) did not significantly differ from virgin females (ΔOD/h 0.65 [0.59–0.70]) (Table 1). There was also no significant effect on phenoloxidase activity of generation, female size, female genotype, or the interaction between female genotype and mating status (Table 1 and Fig. 3A).
A Virgin and mated females from three genotypic backgrounds (E, F, and I). For this analysis, all matings were within genotypic backgrounds. B Females from the three genotypic backgrounds (E, F, and I) mated to male genotypes (E, F, and I) to produce all possible own and other line-mating combinations.
The number of circulating hemocytes of a female was significantly affected by the interaction between mating status and female genotype (Fig. 4A and Table 1). Specifically, while mating did not affect hemocyte number in lines E and F, there was a significant reduction in hemocytes following mating for females from line I (Fig. 4A).
A Virgin and mated females from three genotypic backgrounds (E, F, and I). For this analysis, all matings were within genotypic backgrounds. Pairwise brackets in A indicate significant differences between female mating status within a female genotypic background (Tukey HSD, ***p < 0.001). B Females from the three genotypic backgrounds (E, F, and I) mated to male genotypes (E, F, and I) to produce all possible own and other line-mating combinations. Pairwise brackets in B indicate significant differences between male-mate genotypes within a female genotypic background (Tukey HSD, *p < 0.05, ***p < 0.001).
For hemocyte microaggregations, there was no significant effect of generation, female body size, or the interaction between mating status and female genotype, and no significant difference in numbers of microaggregations between virgin females (estimated marginal mean [95% C.I.] = 0.76 [0.51–1.15]) and mated females (1.11 [0.77–1.60]) (Table 1 and Fig. 5A). However, female genotype significantly affected microaggregations (Table 1), with females from line E (1.40 [0.89–2.20]) and line F (0.55 [0.32–0.95]) significantly differing (Tukey HSD, p < 0.05), and females from line I being intermediate (1.08 [0.71–1.66]).
A Virgin and mated females from three genotypic backgrounds (E, F, and I). For this analysis, all matings were within genotypic backgrounds. Pairwise brackets in A between female genotypic backgrounds indicate significant differences between them (Tukey HSD, *p < 0.05), independent of mating status. B Females from the three genotypic backgrounds (E, F, and I) mated to male genotypes (E, F, and I) to produce all possible own and other line-mating combinations. Pairwise brackets below the legend of B indicate significant differences between male-mate genotypes (Tukey HSD, **p < 0.01), independent of female genotype.
The influence of male and female genotypes on post-mating female immunity
In mated females, within the fully reciprocal mating design across female and male lines (Fig. 1), there was no significant effect on female antibacterial activity of generation, female genotype, male genotype, or the interaction of male and female genotypes (Table 2 and Fig. 2B). There was also no significant effect on phenoloxidase activity of generation, female size, female genotype, male genotype, or the interaction between male and female genotypes (Table 2 and Fig. 3B). However, as in the analyses of mating status, there was a significant effect of female body size on humoral antibacterial activity, with larger females having greater activity (β = 0.145, Table 2).
The number of circulating hemocytes of mated females was significantly affected by a male-genotype-by-female-genotype interaction (Fig. 4B and Table 2). Hemocyte numbers were invariant for females in line E, independent of the male-mate genotype. However, for females from lines F and I, post-mating numbers of circulating hemocytes were significantly reduced in those females mated to line E and I males, relative to those mated with line-F males (Fig. 4B). As expected from this pattern, there was no significant difference in hemocyte counts between within-line matings categorized as “self” and between-line matings classified as other (LRT X21 = 0.424, p = 0.5148).
There was no significant male–female genotype-by-genotype interaction on hemocyte microaggregations of females post mating, nor any significant effect of generation, female size, or female genotype (Table 2 and Fig. 5B). However, the male-mate genotype significantly affected female microaggregations (Table 2), with females mated to males from line E (1.98 [1.33–2.96]) and those mated to males of line F (0.78 [0.49–1.26]) significantly differing (Tukey HSD, p < 0.01), and with females mated to males from line-I intermediate (1.25 [0.82–1.90]).
Discussion
Pre- and post-copulatory interactions may evolve as an interacting phenotype depending on genetic variation for the traits in males and females (Edward et al. 2014; Hall et al. 2013). In our study, such traits of time to female mounting, indicating mate acceptance, and nuptial gift feeding were affected by female but not male genotype. Based on these reciprocal crosses, this could indicate a reduced potential for ongoing sexual antagonistic coevolution in these traits (Edward et al. 2014). However, the main interacting phenotype of interest in our study was female immunity post mating. We demonstrate that mating per se affects a component of female decorated cricket cellular immunity depending on genotypic background, and that differences in post-mating effects in this measure are the result of an interaction between male and female genotypes. Specifically, the depletion in the number of circulating hemocytes in mated females was contingent on an interaction between her genotype and that of her mate. In addition to hemocyte numbers, in our analysis of only mated females, we found evidence that the mating male’s genotype may also influence the presence of hemocyte microaggregations. While we found an influence of mating and interactions between mate genotypes on components of female cellular immunity, we did not uncover any such effects for humoral immunity.
Sexual conflict will result if male and female interests do not match when it comes to the extent of post-mating changes to female immunity (Fedorka et al. 2007). Subsequent sexually antagonistic coevolution could maintain genetic variation in male effects on female immunity, and similarly, female susceptibility to these effects. A following prediction is that the change in female immune profiles post mating will be determined by the specific genotypic combination of the mating partners (Short and Lazzaro 2010). The only prior studies in insects assaying for an interaction between male and female genotypes in determining changes to female post-mating immunity have been in Drosophila (Delbare et al. 2017, Fricke et al. 2020, Short and Lazzaro 2010). These studies have provided mixed results, suggesting that the hypothesis that sexual conflict underlies post-mating changes to female immunity is not universal in this system (Short and Lazzaro 2010). Despite only investigating reciprocal crosses between three genotypic backgrounds in our study on decorated crickets, the male-by-female-genotype interaction on circulating hemocytes of females post mating is consistent with divergence of genotypes of males in their ability to affect female immunity and divergence of female genotypes in their ability to resist. This is predicted if changes to female immunity post mating result from evolution under sexual conflict. Females of line E were universally resistant to mating-induced reductions in hemocyte numbers that were seen in females of lines F and I after matings with E and I males. On the other hand, this effect was not apparent in females of any line mated to males of line F, suggesting potential absence of this effect or resistance to physiological perturbation by F males across all female genotypes tested.
An influence of mating on female immunity has been shown in several studies in insects (Delbare et al. 2017; Lawniczak et al. 2007; Oku et al. 2019; Schwenke et al. 2016), including in other cricket species (Bascuñán-García et al. 2010; Fedorka and Zuk 2005; Fedorka et al. 2004; Shoemaker et al. 2006; Worthington and Kelly 2016). In decorated crickets, we found hemocyte number, a component of measured baseline female cellular immunity, to be more reactive to mating and mating-by-genotype effects than the measured components of female humoral immunity. Our demonstration of a reduction in hemocyte numbers in mated females within certain mate genotype combinations mirrors reductions in hemocyte loads in female-striped ground crickets, Allonemobius socius, exposed to increasing mating effort (Fedorka and Zuk 2005; Fedorka et al. 2004). However, the absence of any significant influence of mating on baseline potential female humoral immunity is in contrast to other studies in insects that demonstrate the effects of mating on these components, including phenoloxidase (Castella et al. 2009; Fedorka et al. 2004; Rolff and Siva-Jothy 2002) and antibacterial activity or expression of AMPs (Barribeau and Schmid-Hempel 2017; Castella et al. 2009; Peng et al. 2005). This suggests that while mating effects on female immunity may be widespread across insects, the components of female immunity affected may be specific to the system, genotypes, or populations involved. The difference in decorated crickets may result from the intrinsic properties of the assayed immune components. In a prior study, an immune challenge induced changes in hemocyte numbers but not antibacterial activity (Duffield et al. 2018), and such properties may affect their differential reactions in the face of physiological perturbation following mating. A lack of consistency in mating effects within specific components of female immunity across insect taxa should be taken into consideration if predictions are not met for one component, given the multifaceted nature of insect immunity (Gillespie et al. 1997).
Further studies are required to uncover the mechanistic underpinnings of the male-genotype-by-female-genotype effects on female post-mating hemocyte loads shown here. Seminal proteins contained in the ejaculate and conversely the female receptors and responses to these are an obvious candidate, being known to orchestrate a cascade of reproductive physiological effects in females of other insect taxa (Perry et al. 2013). Other transferred compounds could also contribute to the effect of mating on female immunity. For example, in the field cricket, G. texensis, prostaglandin, an eicosanoid known to affect hemocyte activation, migration, and microaggregation (Miller et al. 1994; Stanley and Kim 2014), is transferred to females via the male ejaculate, together with hypothesized immune-boosting effects (Worthington and Kelly 2016; Worthington et al. 2015). In contrast to seminal proteins, molecules such as eicosanoids are less likely to vary qualitatively across genotypes, with male lines instead differing quantitatively in their production and female lines differing in their receipt and processing. Therefore, such molecules could plausibly explain variation in male or female genotypes but not genotype-by-genotype effects. The same is true of sexually transmitted microbes that may be commonly associated with insect genitalia (Otti 2015). In the decorated cricket study system, we cannot rule out that components of the nuptial gifts of males (i.e., spermatophylax) orally ingested by females at mating play a role. A recent proteomic investigation of the decorated cricket spermatophylax identified 30 different proteins, 18 of which are encoded by genes expressed in the male accessory glands with uncharacterized functions but hypothesized potential to affect female physiology (Pauchet et al. 2015). Comparable to seminal proteins, differences in male spermatophylax proteins and female responses to them across genotypes have the potential to underlie genotype-by-genotype effects. There was a significant influence of female genotype on the spermatophylax feeding time of females, but longer feeding times also lead to greater ejaculate transfer (Sakaluk 1984). Disentangling the factors responsible for changes in female immunity in this system, including the individual effects of seminal and spermatophylax proteins, represents an interesting direction of future study.
Regardless of the underlying proximate mechanism, the genotype-dependent reduction in hemocytes as a component of cellular immunity is suggestive of an effect that will affect female lifetime fitness. However, it should be noted that in this study, we measured baseline potential immunity in the absence of activation by an infectious agent. Although hemocyte loads in some insect species are positively correlated with parasitoid resistance (Kacsoh and Schlenke 2012) and hemocytes have important roles in antiparasite responses (Kwon and Smith 2019; Ramirez et al. 2014), the link between immunity and realized infection outcomes may not always be clear (Adamo 2004; Shoemaker et al. 2006). In decorated crickets, further studies are needed to link hemocyte reductions post mating with any impact on female resistance to infection. However, in addition to well-documented direct roles in fighting infection (Lavine and Strand 2002), insect hemocytes are involved in other physiological processes, including wound repair (Theopold et al. 2004) and stress responses (Azad et al. 2011). Therefore, the genotype-dependent demonstration of female hemocyte depletion may have farther-reaching consequences for female lifetime fitness beyond fighting infection.
In conclusion, while humoral components of female post-mating baseline immunity were invariant, we show that a component cellular immunity of female decorated crickets post mating is altered as a result of a male–female genotype-by-genotype interaction. This could result from sexual conflict affecting the evolution of female post-mating immune suppression. Future studies are required to determine whether male ejaculates have evolved specifically to manipulate female immunity in G. sigillatus as an adaptation favored in the context of sexual conflict, or if levels of female immunity after mating are merely an incidental effect of other manipulations of female physiology. However, these effects of mating on female immunity, be they direct manipulations by males or side effects of other processes, will influence the selection landscape for females if they affect their resistance to infection.
Data availability
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.547d7wm6n.
References
Adamo SA (2004) Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J Insect Physiol 50:209–216
Alcock J (1994) Postinsemination associations between males and females in insects: the mate-guarding hypothesis. Annu Rev Entomol 39:1–21
Archer CR, Zajitschek F, Sakaluk SK, Royle NJ, Hunt J (2012) Sexual selection affects the evolution of lifespan and ageing in the decorated cricket Gryllodes sigillatus. Evolution 66:3088–3100
Arnqvist G (1988) Mate guarding and sperm displacement in the water strider Gerris lateralis Schumm. (Heteroptera: Gerridae). Freshw Biol 19:269–274
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Arnqvist G, Rowe L (2002) Antagonistic coevolution between the sexes in a group of insects. Nature 415:787–789
Avila FW, Sirot LK, Laflamme BA, Rubinstein CD, Wolfner MF (2010) Insect seminal fluid proteins: Identification and function. Annu Rev Entomol 56:21–40
Azad P, Ryu J, Haddad GG (2011) Distinct role of Hsp70 in Drosophila hemocytes during severe hypoxia. Free Radic Biol Med 51:530–538
Baer B, Morgan ED, Schmid-Hempel P (2001) A nonspecific fatty acid within the bumblebee mating plug prevents females from remating. Proc Natl Acad Sci U S A 98:3926–3928
Barribeau SM, Schmid-Hempel P (2017) Sexual healing: mating induces a protective immune response in bumblebees. J Evol Biol 30:202–209
Bascuñán-García AP, Lara C, Córdoba-Aguilar A (2010) Immune investment impairs growth, female reproduction and survival in the house cricket, Acheta domesticus. J Insect Physiol 56:204–211
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48.
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Burpee DM, Sakaluk SK (1993) Repeated matings offset costs of reproduction in female crickets. Evol Ecol 7:240–250
Castella G, Christe P, Chapuisat (2009) Mating triggers dynamic immune regulations in wood ant queen. J Evol Biol 22:564–570
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Cordero A (1990) The adaptive significance of the prolonged copulations of the damselfly, Ischnura graellsii (Odonata: Coenagrionidae). Anim Behav 40:43–48
Cordero A (1999) Forced copulations and female contact guarding at a high male density in a calopterygid damselfly. J Insect Behav 12:27–37
Delbare SYN, Chow CY, Wolfner MF, Clark AG (2017) Roles of female and male genotype in post-mating responses in Drosophila melanogaster. J Hered 4:740–753
Dickinson JL, Rutowski RL (1989) The function of the mating plug in the chalcedon checkerspot butterfly. Anim Behav 38:154–162
Dougherty LR, van Lieshout E, McNamara KB, Moschilla JA, Arnqvist G, Simmons LW (2017) Sexual conflict and correlated evolution between male persistence and female resistance traits in the seed beetle Callosobruchus maculatus. Proc R Soc B Biol Sci 284:20170132
Duffield KR, Hampton KJ, Houslay TM, Hunt J, Rapkin J, Sakaluk SK, Sadd BM (2018) Age-dependent variation in the terminal investment threshold in male crickets. Evolution 72:578–589
Duffield KR, Hampton KJ, Houslay TM, Hunt J, Sadd BM, Sakaluk SK (2019) Inbreeding alters context‐dependent reproductive effort and immunity in male crickets. J Evol Biol 32:731–741
Edward DA, Poissant J, Wilson AJ, Chapman T (2014) Sexual conflict and interacting phenotypes: a quantitative genetic analysis of fecundity and copula duration in Drosophila melanogaster. Evolution 68:1651–1660
Eggert AK, Reinhardt K, Sakaluk SK (2003) Linear models for assessing mechanisms of sperm competition: the trouble with transformations. Evolution 57:173–176
Fedorka KM, Zuk M (2005) Sexual conflict and female immune suppression in the cricket, Allonemobious socius. J Evol Biol 18:1515–1522
Fedorka KM, Zuk M, Mousseau TA (2004) Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution 58:2478–2485
Fedorka KM, Linder JE, Winterhalter W, Promislow D (2007) Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc R Soc B Biol Sci 274:1211–1217
Fricke C, Ávila‐Calero S, Armitage SA (2020) Genotypes and their interaction effects on reproduction and mating‐induced immune activation in Drosophila melanogaster. J Evol Biol 33:930–941
Gershman SN, Barnett CA, Pettinger AM, Weddle CB, Hunt J, Sakaluk SK (2010a) Inbred decorated crickets exhibit higher measures of macroparasitic immunity than outbred individuals. Heredity 105:282–289
Gershman SN, Barnett CA, Pettinger AM, Weddle CB, Hunt J, Sakaluk SK (2010b) Give ‘til it hurts: trade-offs between immunity and male reproductive effort in the decorated cricket, Gryllodes sigillatus. J Evol Biol 23:829–839
Gershman SN, Hunt J, Sakaluk SK (2013) Food fight: sexual conflict over free amino acids in the nuptial gifts of male decorated crickets. J Evol Biol 26:693–704
Gershman SN, Mitchell C, Sakaluk SK, Hunt J (2012) Biting off more than you can chew: sexual selection on the free amino acid composition of the spermatophylax in decorated crickets. Proc R Soc B Biol Sci 279:2531–2538
Gillespie JP, Kanost MR, Trenczek T (1997) Biological mediators of insect immunity. Annu Rev Entomol 42:611–654
Gillott C (2003) Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu Rev Entomol 48:163–184
Goenaga J, Yamane T, Rönn J, Arnqvist G (2015) Within-species divergence in the seminal fluid proteome and its effect on male and female reproduction in a beetle. BMC Evol Biol 15:266
González-Santoyo I, Córdoba-Aguilar A (2012) Phenoloxidase: a key component of the insect immune system. Entomol Exp Appl 142:1–16
Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Ravi Ram K, Sirot LK et al (2007) Evolution in the fast lane: Rapidly evolving sex-related genes in Drosophila. Genetics 177:1321–1335
Hall MD, Lailvaux SP, Brooks RC (2013) Sex‐specific evolutionary potential of pre‐and postcopulatory reproductive interactions in the field cricket, Teleogryllus commodus. Evolution 67:1831–1837
Hardin JW, Hilbe, JM (2007) Generalized linear models and extensions. 2nd edn, Stata Press, College Station, Texas
Ivy TM, Sakaluk SK (2005) Polyandry promotes enhanced offspring survival in decorated crickets. Evolution 59:152–159
Ivy TM, Weddle CB, Sakaluk SK (2005) Females use self-referent cues to avoid mating with previous mates. Proc R Soc B Biol Sci 272:2475–2478
Kacsoh BZ, Schlenke TA (2012) High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS ONE 7:e34721
Kerr AM, Gershman SN, Sakaluk SK (2010) Experimentally induced spermatophore production and immune responses reveal a trade-off in crickets. Behav Ecol 21:647–654
Klowden MJ (1999) The check is in the male: Male mosquitoes affect female physiology and behavior. J Am Mosq Contr 15:213–220
Kwon H, Smith RC (2019) Chemical depletion of phagocytic immune cells in Anopheles gambiae reveals dual roles of mosquito hemocytes in anti-Plasmodium immunity. Proc Natl Acad Sci U S A 116:14119–14128
Lawniczak MKN, Barnes AI, Linklater JR, Boone JM, Wigby S, Chapman T (2007) Mating and immunity in invertebrates. Trends Ecol Evol 22:48–55
Lavine MD, Strand MR (2002) Insect hemocytes and their role in immunity. Insect Biochem Mol Biol 32:1295–1309
Lenth, SingmannH, Love J, Buerkner P, Herve M (2020) emmeans: estimated marginal means, aka least-squares means. Release 1.4.5. https://CRAN.R-project.org/package=emmeans
Lung O, Wolfner MF (2001) Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol 31:543–551
Miller JS, Nguyen T, Stanley-Samuelson DW (1994) Eicosanoids mediate insect nodulation responses to bacterial infections. Proc Natl Acad Sci U S A 91:12418–12422
Morrow EH, Innocenti P (2012) Female postmating immune responses, immune system evolution and immunogenic males. Biol Rev 87:631–638
Nappi AJ, Vass E (1993) Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res 6:117–126
Oku K, Price TAR, Wedell N (2019) Does mating negatively affect female immune defences in insects? Anim Biol 69:117–136
Otti O (2015) Genitalia‐associated microbes in insects. Insect Sci 22:325–339
Parker GA, Birkhead TR (2013) Polyandry: the history of a revolution. Philos Trans R Soc Lond B Biol Sci 368:1–13
Pauchet Y, Wielsch N, Wilkinson PA, Sakaluk SK, Svatoš A, ffrench-Constant RH et al (2015) What’s in the gift? Towards a molecular dissection of nuptial feeding in a cricket. PLoS ONE 10:e0140191
Peng J, Zipperlen P, Kubli E (2005) Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr Biol 15:1690–1694
Perry JC, Siro L, Wigby S (2013) The seminal symphony: how to compose an ejaculate. Trends Ecol Evol 28:414–422
Ramirez JL, Garver LS, Brayner FA, Alves LC, Rodrigues J, Molina-Cruz A et al (2014) The role of hemocytes in Anopheles gambiae antiplasmodial immunity. J Innate Immun 6:119–128
Ravi Ram K, Wolfner MF (2007) Seminal influences: Drosophila acps and the molecular interplay between males and females during reproduction. Integr Comp Biol 47:427–445
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rice WR, Holland B (1997) The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific red queen. Behav Ecol Sociobiol 41:1–10
Rolff J, Siva-Jothy MT (2002) Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc Natl Acad Sci U S A 99:9916–9918
Rowe L, Arnqvist G (2002) Sexually antagonistic coevolution in a mating system: cominbing experimental and comparative approaches to address evolutionary processes. Evolution 56:754–767
Sakaluk SK (1984) Male crickets feed females to ensure complete sperm transfer. Science 223:609–610
Sakaluk SK (1986) Sperm competition and the evolution of nuptial feeding behavior in the cricket, Gryllodes supplicans (Walker). Evolution 40:584–593
Sakaluk SK (1987) Reproductive behaviour of the decorated cricket, Gryllodes supplicans (Orthoptera: Gryllidae): calling schedules, spatial distribution, and mating. Behaviour 100:202–225
Sakaluk SK (1991) Post-copulatory mate guarding in decorated crickets. Anim Behav 41:207–216
Sakaluk SK (2000) Sensory exploitation as an evolutionary origin to nuptial food gifts in insects. Proc R Soc Lond B Biol Sci 267:339–343
Sakaluk SK, Avery RL, Weddle CB (2006) Cryptic sexual conflict in gift-giving insects: chasing the chase-away. Am Nat 167:94–104
Sakaluk SK, Duffield KR, Rapkin J, Sadd BM, Hunt J (2019) The troublesome gift: the spermatophylax as a purveyor of sexual conflict and coercion in crickets. Adv Stud Behav 51:1–30
Sakaluk SK, Eggert AK (1996) Female control of sperm transfer and intraspecific variation in sperm precedence: antecedents to the evolution of a courtship food gift. Evolution 50:694–703
Sakaluk SK, Schaus JM, Eggert AK, Snedden WA, Brady PL (2002) Polyandry and fitness of offspring reared under varying nutritional stress in decorated crickets. Evolution 56:1999–2007
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schneider PM (1985) Purification and properties of three lysozymes from hemolymph of the cricket, Gryllus bimaculatus (De Geer). Insect Biochem 15:463–470
Schwenke RA, Lazzaro BP, Wolfner MF (2016) Reproduction-immunity trade-offs in insects. Annu Rev Entomol 61:239–256
Sherman KJ (1983) The adaptive significance of postcopulatory mate guarding in a dragonfly, Pachydiplax longipennis. Anim Behav 31:1107–1115
Shoemaker KL, Parsons NM, Adamo SA (2006) Mating enhances parasite resistance in the cricket Gryllus texensis. Anim Behav 71:371–380
Short SM, Lazzaro BP (2010) Female and male genetic contributions to post-mating immune defence in female Drosophila melanogaster. Proc R Soc B Biol Sci 277:3649–3657
Soderhall K, Cerenius L (1998) Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol 10:23–28
Stanley D, Kim Y (2014) Eicosanoid signaling in insects: from discovery to plant protection. Crit Rev Plant Sci 33:20–63
Sugumaran M, Nellaiappan K, Valivittan K (2000) A new mechanism for the control of phenoloxidase activity: inhibition and complex formation with quinone isomerase. Arch Biochem Biophys 379:252–260
Theopold U, Schmidt O, Söderhäll K, Dushay MS (2004) Coagulation in arthropods: defence, wound closure and healing. Trends Immunol 25:289–294
Therneau T (2020) A package for survival analysis in R. Release 3.1-11. https://CRAN.R-project.org/package=survival
Warwick S, Vahed K, Raubenheimer D, Simpson SJ (2009) Free amino acids as phagostimulants in cricket nuptial gifts: support for the “candymaker” hypothesis. Biol Lett 5:194–196
Wigby S, Chapman T (2005) Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol 15:316–321
Wolfner MF (1997) Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem Mol Biol 27:179–192
Worthington AM, Jurenka RA, Kelly CD (2015) Mating for male-derived prostaglandin: a functional explanation for the increased fecundity of mated female crickets? J Exp Biol 218:2720–2727
Worthington AM, Kelly CD (2016) Females gain survival benefits from immune-boosting ejaculates. Evolution 70:928–933
Yi HY, Chowdhury M, Huang YD, Yu XQ (2014) Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol 98:5807–5822
Zhong W, Priest NK, McClure CD, Evans CR, Mlynski DT, Immonen E et al (2013) Immune anticipation of mating in Drosophila: turandot m promotes immunity against sexually transmitted fungal infections. Proc R Soc B Biol Sci 280:1–9
Acknowledgements
This research was funded by a grant from the National Science Foundation to SKS, BMS, and JH (IOS 16-54028), a grant from the Australian Research Council to JH (DP180101708), a grant from the National Institutes of Health to BMS (1R15GM129681-01), research grants from the Beta Lambda Chapter of the Phi Sigma Biological Honor Society, and the Animal Behaviour Student Research Grant to KJH, and an E.L. Mockford and C.F. Thompson Summer Research Fellowship to KJH During the preparation of this paper, SKS was supported by a scholarship from the Deutscher Akademischer Austauschdienst under the auspices of the Research Stays for University Academics and Scientists program. We thank the editor Gerald Heckel and three anonymous reviewers for their insightful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Gerald Heckel
Supplementary information
Rights and permissions
About this article
Cite this article
Hampton, K.J., Duffield, K.R., Hunt, J. et al. Male and female genotype and a genotype-by-genotype interaction mediate the effects of mating on cellular but not humoral immunity in female decorated crickets. Heredity 126, 477–490 (2021). https://doi.org/10.1038/s41437-020-00384-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41437-020-00384-8