ABSTRACT
Colorectal cancer (CRC) is the fourth most frequently diagnosed cancer and 30% of all cases of CRC are believed to have a familial component and up to one-third of these (10%) are hereditary. Pathogenic germline variants in multiple genes have been associated with predisposition to hereditary CRC or polyposis. Lynch syndrome (LS) is the most common hereditary CRC syndrome, caused by variants in the mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PMS2 and is inherited in a dominant manner. Heritable conditions associated with colonic polyposis include familial adenomatous polyposis (FAP) associated with APC pathogenic variants, MUTYH-associated polyposis (MAP) caused by biallelic MUTYH pathogenic variants, and polymerase proofreading–associated polyposis (PPAP) caused by POLE or POLD1 pathogenic variants. Given the overlapping phenotypes of the cancer syndromes along with the limited sensitivity of using clinical criteria alone, a multigene panel testing approach to diagnose these conditions using next-generation sequencing (NGS) is effective and efficient. This technical standard is not recommended for use in the clinic for patient evaluation. Please refer to National Comprehensive Cancer Network (NCCN) clinical practice guidelines to determine an appropriate testing strategy and guide medical screening and management. This 2021 edition of the American College of Medical Genetics and Genomics (ACMG) technical standard supersedes the 2013 edition on this topic.
Similar content being viewed by others
INTRODUCTION
Colorectal cancer (CRC) is the fourth most frequently diagnosed cancer and the second leading cause of cancer death in the United States. The incidence of CRC has been reported by the Centers for Disease Control and Prevention (CDC) as 40 per 100,000 persons. In 2016, an estimated 95,520 new cases of colon cancer and 39,910 new cases of rectal cancer were reported in the United States.1 That same year, an estimated 50,260 people died from CRCs. Most cases of CRC are sporadic, but familial cancer syndromes are also common in this disease.1 Approximately 10% of all cases of CRCs are believed to be hereditary, and up to 30% are thought to have a familial component, including genetic background and common environmental risk factors.1,2
Pathogenic germline variants in multiple genes have been implicated in inherited CRC or polyposis.3 Colorectal polyps are considered precursors to CRC. Well-characterized conditions associated with colonic polyposis include familial adenomatous polyposis (FAP) and MUTYH-associated polyposis (MAP). FAP is an autosomal dominant disorder characterized by innumerable colonic adenomatous polyps and pathogenic germline variants in APC. MAP is an autosomal recessive condition characterized by an attenuated phenotype relative to FAP; affected patients may have fewer adenomatous polyps and are often diagnosed later in life compared to FAP.
Lynch syndrome (LS), previously known as hereditary nonpolyposis colorectal cancer (HNPCC), is the most common hereditary CRC syndrome. It is typically recognized as an assemblage of associated cancers characterized by microsatellite instability (MSI-High [H]). Universal screening for LS via MSI and/or immunohistochemistry (IHC) for mismatch repair proteins (MMR protein complex: MLH1, MSH2, MSH6, and PMS2) in individuals with CRC or endometrial cancer is recommended, and additional testing criteria may also be used to identify at-risk individuals.2 Pathogenic variants in the MMR genes are implicated in LS.
With the adoption of massively parallel sequencing (also known as next-generation sequencing [NGS]) by many clinical laboratories, it is now possible for timely, cost-effective analysis of multiple genes associated with inherited polyposis and/or CRC. Given the overlapping phenotypes of high-penetrance cancer syndromes along with the limited sensitivity of using clinical criteria alone, a multigene panel testing approach for the diagnosis of these conditions is effective and efficient. This technical standard is not recommended for use in the clinic for patient evaluation. Please refer to National Comprehensive Cancer Network (NCCN) clinical practice guidelines2 to determine an appropriate testing strategy and guide medical screening and management. To aid clinical laboratories in focusing testing on the most clinically significant genes, the ClinGen Hereditary Colorectal Cancer and Polyposis Susceptibility Gene Curation Panel reviews the gene–disease validity for genes included on multigene panels for hereditary CRC or polyposis.3 This Technical Standards document has been updated to include considerations related to multigene panel testing for hereditary CRC and polyposis. The standards herein are limited to genetic testing for inherited CRC and/or polyposis.
Methodology
These technical laboratory standards were informed by a review of the literature and current guidelines. Resources consulted included PubMed, ClinVar database, NCCN guidelines; revised Bethesda guideline for HNPCC and microsatellite instability, gene–disease associations curated by the Clinical Genome Resources (ClinGen) Colon Cancer and Polyposis Gene Curation Expert Panel, and relevant American College of Medical Genetics and Genomics (ACMG), Association for Molecular Pathology (AMP), and College of American Pathologists (CAP) guidelines. The workgroup members also used their expert opinion and empirical data to inform their recommendations. The ACMG Laboratory QA Committee reviewed the documents providing further input on the content, and a final draft was presented to the ACMG Board of Directors for review and approval to post on the ACMG website for member comments. Upon posting to the ACMG website, an email and link were sent to all ACMG members inviting participation in the 30-day open comment process. All members’ comments and additional evidence received were assessed by the authors, and these recommendations were incorporated into the document as deemed appropriate. Member comments and author responses were reviewed by representatives of the ACMG Laboratory QA Committee and the ACMG Board of Directors. The final document was approved for publication by the ACMG Board of Directors.
LYNCH SYNDROME (PREVIOUSLY HEREDITARY NONPOLYPOSIS COLORECTAL CANCER [HNPCC])
Background on Lynch syndrome
LS is the most common form of inherited colorectal cancer. LS is an autosomal dominant disease, with a population incidence of approximately 1 in 1,000, and is responsible for approximately 1–3% of all colon cancer cases. It is typically recognized as an assemblage of cancers characterized by microsatellite instability (MSI-High [H]), genetic heterogeneity, and caused by germline variants in the four mismatch repair genes: MLH1, MSH2, MSH6, and PMS2. In addition, EPCAM, which is upstream of MSH2, is also implicated due to large deletions of its 3’ end or 3’ untranslated region (UTR) that lead to hypermethylation of the MSH2 promoter and loss of MSH2 expression.4,5
Brief clinical description
Patients with LS have up to an 80% lifetime risk of developing colon cancer and, in women, a 60% lifetime risk of developing endometrial carcinoma. Affected individuals are also at greater risk for other cancers, such as stomach, ovarian, small bowel, biliary, renal pelvis, and ureteral cancers. In contrast to FAP, the number of polyps seen in patients with LS is at or slightly above the rate in the general population, but these precursor lesions progress more rapidly through the stages of carcinogenesis. Relative to sporadic CRC, adenomas and carcinomas in LS occur predominantly in the proximal colon.
The average age of onset for colorectal cancer in LS is 44 years, approximately 20 years earlier than the age of diagnosis of individuals with sporadic colorectal cancer. The age of diagnosis of LS-associated endometrial cancer is 46–62 years. LS-associated ovarian cancers have a mean age of diagnosis of 42.5 years; however, approximately 30% of women who develop ovarian cancer are diagnosed before the age of 40. Among women with LS who develop both colon cancer and endometrial cancer, 50% present first with endometrial cancer.
The first guidelines for clinical diagnosis of LS were the Amsterdam criteria, developed in 1990. However, these guidelines only identified LS in approximately 60% of cases. This lack of sensitivity led to a revision (Amsterdam II criteria) that accounts for the presence of extracolonic cancers and reaches a detection sensitivity of around 80%.6 Currently, most diagnoses stem from the National Cancer Institute’s Bethesda Guidelines that were developed in 1997 to advise on testing of tumors for MSI when CRC occurred under age 50, a synchronous (e.g., more than one primary colorectal cancer is detected at initial presentation) or metachronous (e.g., new colorectal cancers are diagnosed more than six months after surgery for the primary colorectal tumor[s]) colon cancer or other related cancer was present, and/or there was a significant family history.7,8
Most LS patients inherit a variant in one of the mismatch repair (MMR) genes from a parent; however, de novo pathogenic variants have been reported. Cancer development in LS occurs at variable ages, though frequently younger than expected based on the incidence in the general population. Lynch syndrome shows incomplete penetrance.9 Rare, biallelic, inherited MMR variants have been reported in MLH1, MSH2, MSH6, and PMS2 and are associated with constitutional mismatch repair deficiency syndrome (CMMRDS).10,11,12
Gene symbol, chromosome locus, MIM, and transcript numbers
LS (MIM 120435) is a genetically heterogeneous disease caused by pathogenic variants in the following mismatch repair genes and EPCAM:
MLH1: MutL, E. coli, homolog of, 1; located on chromosome 3p21.3 (MIM 120436), NM_000249.5,
MSH2: MutS, E. coli, homolog of, 2; located on chromosome 2p22-p21 (MIM 609309), NM_000251.1,
MSH6: MutS, E. coli, homolog of, 6; located on chromosome 2p16 (MIM 600678), NM_000179.2,
PMS2: Postmeiotic segregation increased S. cerevisiae, 2; located on chromosome 7p22 (MIM 600259), NM_000535.5,
EPCAM: Epithelial cellular adhesion molecule; located on chromosome 2p21 (MIM 185535), NM_002354.2 limited to large pathogenic copy-number variation.
Gene descriptions/normal gene products
MLH1
MLH1 is 57,357 bases in length, consisting of 19 coding exons; the translated protein contains 756 amino acids. The protein MLH1 dimerizes with the protein product of PMS2 to coordinate the binding of other proteins involved in mismatch repair, including the helicases, the protein encoded by EXO1, proliferating cell nuclear antigen (PCNA), single-stranded-DNA binding-protein (RPA), and DNA polymerases.
MSH2
MSH2 is 80,097 bases in length, consisting of 16 coding exons; the translated protein contains 934 amino acids. The MSH2 protein forms a heterodimer with either DNA mismatch repair protein MSH6 or MSH3 and functions to identify mismatches. A sliding clamp model has been put forward to describe the structure of the heterodimer. Mismatches in the DNA are thought to be detected as the clamp slides along the DNA.
MSH6
MSH6 is 23,871 bases in length, consisting of 10 coding exons; the translated protein contains 1,360 amino acids. The MSH6 protein forms a heterodimer with DNA mismatch repair protein MSH2 (see “MSH2” for details on function).
PMS2
PMS2 is 35,867 bases in length, consisting of 15 coding exons; the translated protein contains 862 amino acids. The PMS2 protein dimerizes with the MLH1 protein (see “MLH1” for details on the function of this protein dimer). PMS2 is the only gene in this complex that has several untranslated pseudogenes.13
Function of mismatch repair genes
Mismatch repair (MMR) genes are involved in numerous cellular functions, including:
-
1.
Repairing DNA synthesis errors.
-
2.
Repairing double-strand DNA breaks.
-
3.
Apoptosis.
-
4.
Antirecombination.
-
5.
Destabilization of DNA.
These functions make MMR proteins extremely important in the basic maintenance of the genetic material, the regulation of the cellular cycle, and the development of an effective immune system. When MMR function is lost or defective, there is a decrease in apoptosis, an increase in cell survival, and an increase in damage-induced mutagenesis. These changes provide a selective growth advantage to affected cells, thereby increasing susceptibility to tissue-specific cancers.
EPCAM
EPCAM is 42,444 bases in length, consisting of 9 coding exons; the translated protein contains 314 amino acids. The epithelial cell adhesion molecule (EPCAM) encodes a carcinoma-associated antigen and is a member of a family that includes at least two type I membrane proteins. This antigen is expressed on most normal epithelial cells and gastrointestinal carcinomas, and functions as a homotypic calcium-independent cell adhesion molecule. The EPCAM gene is located upstream of the MSH2 gene. The pathogenic variants in EPCAM are not directly causative of LS by themselves, rather germline deletions involving its 3’ portion or its 3’ untranslated region allow for subsequent epigenetic silencing of MSH2, causing LS. Hypermethylation of MSH2 gives rise to microsatellite instability and LS.
Pathogenic variant/abnormal gene product
LS is caused by a pathogenic germline variant in a MMR gene. The causative genes identified to date are noted above. Among these, approximately 50% of the pathogenic variants are in MLH1 and 40% in MSH2. Pathogenic variants in MSH6 account for 7–10% of families with LS, and variants in PMS2 are responsible for fewer than 5% of LS families. These genes cooperatively participate to repair nucleotide mismatch errors arising in DNA replication, and deficiencies in any one of the repair genes can lead to LS. In somatic tumor tissue, the pathogenic variants in the mismatch repair genes result in high levels of microsatellite instability.14,15,16,17,18,19 EPCAM deletions have been reported in 1–3% of LS cases.20,21 Although rare, copy-number neutral pathogenic inversions/structural rearrangements in MSH2, MLH1, and PMS2 have been described and were noted to explain some families with previously unexplained LS and microsatellite unstable carcinomas.22,23,24
Spectrum, prevalence, and ethnic association of common pathogenic variants
Pathogenic variants in the MMR genes have been observed in all ethnic groups. There are more than 15,000 different variants (ClinVar, https://www.ncbi.nlm.nih.gov/clinvar/) identified in the four MMR genes. Pathogenic variants in the MMR genes can be missense, nonsense, small insertions or deletions, splice site, or regulatory variants; all EPCAM variants associated with LS are large deletions that extend to or include the MSH2 gene. Single-nucleotide variants and small indels in the EPCAM gene are not associated with inherited CRC (MIM 185535). Large deletions account for 5–10% of pathogenic MLH1 variants, greater than 20% of pathogenic MSH2 variants, less than 5% of pathogenic MSH6 variants, and an undefined percentage of pathogenic variants in PMS2. There are few mutation hotspots in MMR genes. The splice site variant in intron 5 of MSH2, c.942+3A>T, has been repeatedly seen in different racial groups, including Black, White and Asian populations. The MSH2 p.Ala636Pro variant has been found in 0.59% of the Ashkenazi Jewish population with colorectal cancer.25 A deletion of exon 16 in MLH1 is a founder variant detected in 29 families in Finland, and a deletion of exons 1 to 6 in MSH2 is a founder variant in 18,981 individuals in the United States. A germline inversion of exons 1–7 in MSH2 is a cause of unexplained MSH2-type LS.23
Testing criteria for Lynch syndrome
The NCCN recommends universal screening for MMR deficiency for all colorectal and endometrial tumors regardless of age at diagnosis.2 However, in lower resource situations, the revised Bethesda criteria or Amsterdam II criteria may still be useful to help identify CRC patients whose tumors should be tested for MMR deficiency.
-
1.
Patient meets the Amsterdam II criteria (Table 7, Figure 20A, 20B)26 or the revised Bethesda guidelines (Table 8).7 MSI testing or IHC for the causative gene products in the tumor tissue to confirm MSI-H or the absence of mismatch repair proteins.
-
2.
Presence of synchronous or metachronous colorectal cancer or other LS-related tumor regardless of age.
-
3.
Colorectal cancer in an individual under 60 years of age exhibiting tumor-infiltrating lymphocytes.
-
4.
Colorectal cancer at any age, plus colorectal cancer or LS-related tumor diagnosed before the age of 50 in at least one first-degree relative.
-
5.
Colorectal cancer at any age, plus colorectal cancer or LS-related tumor diagnosed at any age in two or more first-degree or second-degree relatives.
Algorithm for testing
A suggested algorithm for LS testing is shown in Fig. 1. This algorithm takes advantage of certain molecular features of a tumor to determine whether it is likely to be associated with LS, and then how best to proceed with the germline analysis. The specific screening approach may be influenced by clinical factors including tumor type, age of cancer onset in individual being tested, and family history of LS-associated cancers.
Since virtually all colorectal cancers associated with LS exhibit MSI, the first step is to test the tumor for deficient MMR, either by polymerase chain reaction (PCR) of microsatellite repeats or IHC for mismatch repair proteins.27,28,29 The estimated specificity and sensitivity of the detection of LS by PCR-based methods for MSI is 90.2% (95% CI, 87.7–92.7%) and 85% (95% CI, 75–92%), respectively.2 There is a 5–15% false-negative rate with MSI testing. The sensitivity and specificity of MMR IHC for CRC is 92–94% and 88–100%, respectively.2 There is a 5–10% false-negative rate with IHC testing. In addition, IHC results can be used to guide the germline analysis (see below) and whether or not BRAF c.1799T>A, p.V600E testing would be informative with regard to a diagnosis of LS. An abnormal result with either the MSI or IHC test should trigger the next set of tests in the algorithm. The NCCN guidelines for LS also review the principles of MMR deficiency testing by different methodologies and propose testing strategies based on tumor testing results.2
Approximately 10–15% of sporadic colorectal cancers also exhibit MSI. The molecular basis for instability in these tumors is most often methylation of the MLH1 promoter, leading to loss of both messenger RNA (mRNA) and protein expression.30,31 MLH1 promoter region “C” is a small proximal region (-248 to -178 relative to the transcription start site), in which the methylation status correlates with MLH1 expression. Therefore, methylation analysis of the MLH1 promoter region can help distinguish between sporadic and inherited MLH1 loss. Furthermore, more than half of sporadic colonic cancers demonstrating MSI-H and loss of MLH1 have the BRAF p.V600E oncogenic variant, which is not seen in LS-associated cancers.32 Detection of BRAF p.V600E consequently may be helpful to assess for sporadic MSI-H colonic cancers; however, this marker is not useful for assessment of non-CRC Lynch-associated cancers. A subset of sporadic MSI-high cases have double/biallelic somatic variants.33
Given the emergence of panel testing using massively parallel sequencing, the cost of this approach is much closer to that of a single-gene test, so using a NGS-based assay may be more cost-efficient than a single-gene approach, even when IHC is used to identify missing proteins. A multigene panel testing approach may also be considered for unaffected family members when no affected relative is available to test, or substituted for tumor IHC/MSI in individuals with a strong family history of Lynch-associated cancers2 (see Fig. 2). The presence of pseudogenes homologous to PMS2 and copy-number neutral structural rearrangements may require additional assays.
Sensitivity and specificity
A combination of sequencing and copy-number analysis can detect 99% of variants in MLH1, MSH2, MSH6, and PMS2. Copy-number assays used to detect EPCAM deletions are approximately 99% sensitive.
Diagnostic testing
Molecular testing that includes sequencing of coding exons, promoter region and relevant areas of the 5’- and 3’-UTR, and deletion/duplication assays by multiple ligation-dependent probe amplification (MLPA) or other techniques for pathogenic germline variants in the MMR genes and EPCAM is used for diagnosis of LS.34 Positive results are considered diagnostic rather than predictive. Penetrance of LS-associated cancers with variants in these genes is less than 100%. Therefore, some individuals with a cancer-predisposing variant in an MMR gene or EPCAM may not develop cancer in their lifetime.35
HEREDITARY COLONIC POLYPOSIS
Background on hereditary colonic polyposis
Colorectal polyps are precursors to CRC. Well-characterized conditions associated with polyposis include familial adenomatous polyposis (FAP) and MUTYH-associated polyposis (MAP). FAP and related phenotypes are caused by pathogenic germline variants in the APC tumor suppressor gene. In accordance with Knudson’s two-hit hypothesis, both alleles of the APC gene are inactivated in tumors, resulting in loss of the functional protein. MAP is inherited in an autosomal recessive manner; thus, biallelic germline MUTYH variants are required for diagnosis of MAP. Additional genes such as POLD1 and POLE have recently been implicated in hereditary colonic polyposis.3 Identification of germline variant(s) associated with hereditary polyposis in an affected individual is useful for confirmation of diagnosis and clinical management of presymptomatic family members.
Brief clinical description of hereditary colonic polyposis phenotypes
FAP is an autosomal dominant disorder that predisposes to colon cancer. The disease is characterized by the presence of a large number of colorectal adenomatous polyps (>100) that begin to form at a mean age of 16 years. Variable features include extracolonic polyps, dental abnormalities, congenital hypertrophy of the retinal pigment epithelium (CHRPE), soft tissue tumors, and desmoid tumors. Other APC-associated polyposis phenotypes include attenuated FAP (AFAP), which is characterized by fewer colonic polyps (average of 30) and occurs later in life (average age of onset around 50 years), and gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS). Approximately 30% of FAP cases result from de novo germline variants in APC so there may not be a prior family history of colonic adenomatous polyps or colorectal carcinoma.
The terms Gardner syndrome and Turcot syndrome were used to describe variants of FAP. Gardner syndrome was historically used to describe colonic adenomatous polyposis accompanied by osteomas and soft tissue tumors, and Turcot syndrome was used to refer to the association of colonic adenomatous polyposis with central nervous system tumors.
MAP is a hereditary autosomal recessive disease caused by biallelic germline variants of the MUTYH gene, which is one of the base excision repair genes. MAP is characterized by the presence of about 10–100 adenomas in the large intestine, although some patients could have 1,000 adenomas or more. The incidence of germline MUTYH variation is approximately 1 in 100, suggesting prevalence of MAP of 1 in 40,000. The penetrance of CRC in individuals with MAP is 43–63% at 60 years of age with a lifetime risk up to 100% in the absence of surveillance.
Polymerase proofreading–associated polyposis (PPAP) is a hereditary autosomal dominant disease caused by pathogenic germline variants in the POLE or POLD1 genes. Many patients have a few dozen colorectal adenomas, while others have been reported to have none. Extracolonic manifestations including duodenal adenomas/cancers and brain tumors have been reported in individuals with PPAP-carrying pathogenic POLE variants, while endometrial cancer, breast cancer, and brain tumors have been associated with pathogenic POLD1 variants.
Gene symbol, chromosome locus, MIM, and transcript numbers
APC:
APC gene; located on chromosome 5q22.2 (MIM 175100), NM_000038.5.
MUTYH: MutY DNA glycosylase; this gene has also been referred to as MYH; located on chromosome 1p34.1 (MIM 604933), NM_001128425.1.
POLD1: Polymerase (DNA directed), delta1, catalytic subunit; located on chromosome 19q13.33 (MIM 174761), NM_002691.3.
POLE: Polymerase, DNA, epsilon; located on chromosome 12q24.33 (MIM 174762), NM_006231.3.
Gene descriptions/normal gene products
APC is 138,742 bases in length, consisting of 17 coding exons, the translated protein contains 2,843 amino acids. The protein belongs to the Wnt signal transduction pathway, and is also associated with cell adhesion and microtubule assembly. Absence of functional APC gene product leads to aberrant transcription of C-myc, Cyclin-D, and other target molecules.
MUTYH is 11,731 bases in length, consisting of 16 coding exons, the translated protein contains 549 amino acids. MUTYH, an adenine-specific DNA glycosylase, removes adenine residues mispaired with 8-oxo-dG or guanine.
POLD1 is 33,815 bases in length, consisting of 27 coding exons, the translated protein contains 1,107 amino acids. The POLD1 encodes the catalytic subunit of DNA polymerase delta that possesses both polymerase and 3’ to 5’ exonuclease activity, and plays a critical role in synthesis of the lagging strand during DNA replication. The catalytic component of the trimeric (Pol-delta3 complex) and tetrameric DNA polymerase delta complexes (Pol-delta4 complex) plays a crucial role in high fidelity genome replication, including in lagging strand synthesis and repair.
POLE is 63,767 bases in length, consisting of 49 coding exons, the translated protein contains 2,286 amino acids. This gene encodes the catalytic subunit of DNA polymerase epsilon. The enzyme is involved in DNA repair and chromosomal DNA replication.
Pathogenic variant spectrum
More than 7,000 different germline APC variants have been characterized in ClinVar. An estimated 87% of causative APC germline variants are sequence changes including nonsense, in-frame insertion/deletion, out-of-frame insertion/deletion, and missense variants, and most of them result in the introduction of a termination codon. Small subsets of missense variants have been functionally characterized to have the potential to predispose to FAP. In addition, ~10–15% of germline variants include gross deletions, duplications, insertions, and complex rearrangements.
There are more than 1,400 germline MUTYH variants documented in ClinVar. However, two missense variants, p.Tyr179Cys and p.Gly396Asp based on NM_001128425.1, account for 70–80% of variants in individuals of Northern European ancestry, with a third variant, c.1437_1439delGGA (also known as 1395delGGA), accounting for around 25% of variants in individuals of Southern European (Mediterranean) ancestry.36,37
There are over 2,000 different germline variants in ClinVar for POLD1; however, only several are asserted to be pathogenic or likely pathogenic in relation to CRC. Cancer-predisposing variants include missense substitutions within the endonuclease domain.
Although there are more than 4,000 germline POLE variants in ClinVar, only one pathogenic variant (p.Leu424Val) is known to predispose to CRC; the remainder of the variants are known to predispose to other cancers, such as breast cancer or cutaneous melanoma, although a role in predisposition to CRC cannot be excluded.
Testing criteria
Testing for hereditary colorectal polyposis should be considered for individuals with any of the following:
-
1.
Personal history of 20 or more cumulative adenomas.
-
2.
Multifocal/bilateral congenital hypertrophy of retinal pigment epithelium (CHRPE).
-
3.
Consider testing if a personal history of
-
(a)
Between 10 and 19 cumulative adenomas,
-
(b)
A desmoid tumor,
-
(c)
Hepatoblastoma,
-
(d)
Cribriform-morular variant of papillary thyroid cancer,
-
(e)
Unilateral CHRPE,
-
(f)
Meets criteria for serrated polyposis syndrome with at least some adenomas.
-
(a)
Algorithm for testing
It is recommended that FAP testing be performed by sequencing of the entire coding region and the splice site boundaries of the APC gene. If no pathogenic variant is detected, then testing for large gene rearrangements should be performed. In addition, sequencing and deletion analysis for MUTYH, POLD1, and POLE are also recommended.
Sensitivity and specificity
Comprehensive analysis of the entire APC gene is necessary for diagnostic testing of FAP. Approximately 87% of APC variants are single-nucleotide polymorphisms (SNPs), small deletions, and insertions, and can be detected by sequencing. The remaining ~10–15% of variants are gross deletions and duplications, which can be detected by MLPA, real-time quantitative PCR analysis, array comparative genomic hybridization, or other methods. The analytical sensitivity of MUTYH, POLD1, and POLE sequencing and deletion detection by MLPA, real-time PCR, or microarray approaches 95–98%.
Diagnostic testing
Molecular testing for variants in the APC, MUTYH, POLD1, and POLE genes is used for diagnostic and presymptomatic testing. A pathogenic variant is detected in approximately 80% of patients with a clinical diagnosis of FAP. The detection of a pathogenic variant is diagnostic of FAP. The penetrance of FAP is virtually 100%.
HEREDITARY COLORECTAL CANCER (HCC) AND POLYPOSIS SUSCEPTIBILITY GENES
Background and brief clinical description of HCC and polyposis
Hereditary CRC syndromes may be broadly classified as those associated with or without colorectal polyposis. Approximately 5–10% of all CRCs are related to highly penetrant pathogenic gene variants associated with recognized hereditary disorders such as LS, FAP, MAP, Peutz–Jeghers syndrome (PJS), juvenile polyposis syndrome (JPS), and other syndromes associated with CRC risk (Li–Fraumeni syndrome and Cowen syndrome/PTEN hamartoma tumor syndrome). It is important to identify actionable genetic alterations to provide optimal clinical management including implementing preventive measures and assessing family members’ risk.
The mode of inheritance of the majority of the 23 genes recommended for inclusion on multigene panels is autosomal dominant (Table 1). Exceptions include autosomal recessive inheritance for ATM, BLM, MSH3, MUTYH, and NTHL1. The potential for increased risk for CRC has been described in heterozygotes of ATM and BLM variants. Rare, biallelic inherited variants in MLH1, MSH2, MSH6, and PMS2 have been reported.
Evaluation of genes associated with HCC and polyposis for inclusion on multigene panel
The evidence for pathogenic variants in a gene causing predisposition to hereditary CRC and/or colonic polyposis was evaluated for 44 genes. Genes and evidence for evaluation were identified by review of the medical literature and guidelines from professional organizations such as the NCCN2 and the Clinical Genome Resource (ClinGen) Clinical Validity Framework Colorectal Cancer working group.3 Overall, 23 genes were determined to have at least “moderate” evidence supporting clinical validity by ClinGen gene curation guidelines38,39 and are recommended for clinical testing when a multigene panel approach is elected (Table 1). Eleven of these genes are considered “high risk” and received “definitive” or “strong” clinical validity classifications. These genes are associated with well-characterized CRC and/or polyposis syndromes, which often have established cancer risks and surveillance guidelines, such as APC, MLH1, and SMAD4. Another ten genes are considered “risk genes” and had “moderate” evidence for CRC and/or polyposis susceptibility; however, specific cancer risk estimates or surveillance guidelines may not be available. Three genes (CDH1, FLCN, and TP53) are associated with defined hereditary cancer predisposition syndromes, which include CRC and/or polyposis as rare manifestations. As it is difficult to separate out specific CRC/polyposis risk, it is reasonable to include these genes routinely on multigene panels for HCC.
The gene–disease associations for several genes were evaluated considering monoallelic variants (e.g., ATM, BLM), biallelic variation (e.g., MUTYH, MSH3), or in relation to specific variant type (e.g., EPCAM 3’ and 3’UTR deletions, GREM1 40 kb upstream duplication, POLD1/POLE variants in endonuclease domains) as appropriate.
Genes evaluated with limited gene–disease validity
Twenty-one genes did not reach “moderate” evidence for gene–disease validity for CRC and/or polyposis at the time of publication;3 however, annual review is recommended as evidence may change over time. The evaluated genes with currently “limited” evidence for CRC and/or polyposis predisposition include BARD1, BRCA1, BUB1, BUB3, CDKN1B, CHEK2, CTNNA1, ENG, EPHX1, EXO1, FAN1, GALNT12, NFKBIZ, PALB2, PMS1, PTPRJ, RNF43, RSP20, SEMA4A, SMARCA4, and XRCC4. Variants in these genes should be interpreted with caution in the assessment of inherited CRC and hereditary polyposis.
Gene description, pathogenic variants mechanism, and variants spectrum for genes included in multigene panel
MLH1, MSH2, MSH6, PMS2, and EPCAM (refer to Lynch Syndrome section),
APC, MUTYH, POLD1, and POLE (refer to Hereditary Colonic Polyposis section),
BMPR1A, PTEN, SMAD4, STK11, ATM, AXIN2, BLM, GREM1, MLH3, MSH3, CDH1, FLCN, TP53 (see Table 2).
Testing criteria
Testing for hereditary CRC or polyposis using a multigene panel should be considered when more than one gene may explain an inherited cancer syndrome.2 This approach may be more cost-effective than single-gene testing or sequential single syndrome testing.
Clinical sensitivity of multigene panel
The clinical sensitivity of a multigene panel for hereditary CRC or polyposis will vary depending on the patient’s phenotype, genes included, and specific testing methodology used. Given the growing number of genes in which germline variation is known to increase susceptibility to CRC or polyposis, diagnostic testing using a multigene panel provides a higher chance of identifying a molecular cause for the cancer. Multigene panel testing should be considered in individuals with CRC where more than one gene may explain the presentation (which is often the case due to the extensive clinical and genetic overlap in CRC and polyposis), colonic polyposis of uncertain histology, adenomatous polyposis, or a suspected hereditary cancer syndrome but family cancer history does not meet established testing guidelines.2 At least 10% of patients with CRC harbor at least one pathogenic germline variant in a susceptibility gene identifiable by multigene panel testing;40 this increases to approximately 20% when considering individuals diagnosed before age 50.1,41 Patients with pathogenic variants in non-LS genes are not currently identified by standard of care, unless features suggestive of a single-gene disorder are present. Importantly, an estimated 7–8% of individuals with CRC tested using a multigene panel will have a detectable pathogenic variant in a non-Lynch susceptibility gene.1,40 An estimated one-third to two-thirds of individuals identified to have a high-penetrance hereditary cancer syndrome using multigene panel testing did not meet testing criteria for the gene in which they have been found to harbor a pathogenic variant.1,40,41 Despite the benefits of multigene panel testing, use of a multigene panel results in an estimated 3–4% chance of identifying pathogenic variants for which clinical management is uncertain given the inclusion of “risk genes” of intermediate penetrance, as well as a 17–38% chance of detecting variants of uncertain significance that are not actionable.2 If not correctly interpreted, patients identified to have such variants could be subjected to overtreatment or overscreening.2
METHODOLOGICAL CONSIDERATIONS
All general guidelines for polymerase chain reaction (PCR) and DNA sequencing in the ACMG Technical Standards for Clinical Genetics Laboratories apply (www.acmg.net). The following additional details are relevant to molecular testing performed for LS, hereditary colonic polyposis and HCC.
Methodology for Lynch syndrome
Sample requirement and processing
Performing MSI and IHC testing requires tumor tissue. Either fresh-frozen tissue or a formalin-fixed paraffin-embedded (FFPE) tissue block can be used. Corresponding normal tissue is needed for some MSI assays. DNA is extracted for MSI. DNA extracted from a blood sample can be used as a representative normal tissue from the patient.
For germline variant detection in the MMR genes by Sanger sequencing, NGS, MLPA, or comparative genomic hybridization (CGH) array, DNA extracted from a blood sample is preferred for germline variant assessment.
Immunohistochemistry
IHC is performed for all four MMR proteins: MLH1, MSH2, MSH6, and PMS2.42 After performing hematoxylin and eosin (H&E) staining on sections from FFPE blocks, the sections are analyzed for the expression of MMR proteins. Approaches using only two instead of all four immunostains may lead to missed cases of LS.43
Detection of MSI by PCR
Methods for MSI determination by PCR and IHC have been described (refer to the ACMG Technical Standards for Clinical Genetics Laboratories for details). With respect to PCR, a panel of five mononucleotide microsatellite repeats (BAT-25, BAT-26, MONO-27, NR-21, NR-24) and two pentanucleotide repeats (used for specimen identification) is recommended and demonstrates improved sensitivity and specificity over the original NCI Bethesda panel. If 40% or more of the repeats are unstable, a tumor is classified as MSI-H. If some, but fewer than 40%, of repeats are unstable, a tumor is classified as MSI-low (MSI-L), and if no repeats are unstable, a tumor is classified as microsatellite stable (MSS). MSI can now also be detected using NGS methodologies.44
BRAF p.Val600Glu (p.V600E) variant
The BRAF p.V600E missense variant has been shown to be associated with sporadic CRC. This variant can be detected using a targeted variant detection technique, such as allele-specific primer extension (ASPE), or real-time PCR or as part of a NGS panel. The presence of the BRAF p.V600E variant and MLH1 promoter methylation are strong predictors of sporadic microsatellite instability in CRC. BRAF p.V600E variant has shown lack of association with sporadic endometrial tumors.
MLH1 promoter methylation region “C”
MLH1 promoter methylation region “C” is considered when a tumor shows MLH1 loss and microsatellite instability. Bisulfite modification with real-time (quantitative) methylation specific PCR (MSP) analysis to detect the methylated and unmethylated allele is the common method used.45,46 Refer to the ACMG Technical Standards for Clinical Genetics Laboratories for details.
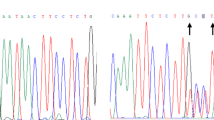
Detection of germline variants in Lynch or polyposis-associated genes by Sanger sequencing
PCR amplification is performed on all coding exons and intron/exon boundaries of MMR genes MLH1, MSH2, MSH6, and PMS2 in patients’ genomic DNA. IHC and MSI results may help narrow down the sequencing to one or two genes of the MMR complex. The same considerations apply to the hereditary colonic polyposis genes: APC, MUTYH, POLD1, and POLE.
Due to the high homology of the PMS2 functional gene and pseudogenes, it is difficult to find PCR and/or sequencing primer binding sites, which allow only amplifying and sequencing of the functional gene.47 Long-range PCR using functional gene specific primers can overcome this problem.
Methods for detecting copy-number variations (deletion and duplication analysis)
The main method to detect these large deletions and duplications is MLPA. With technical advances, NGS can also detect copy-number variation so a separate assay such as MLPA may not be needed. Deletion of two or more sequential probes detected by MLPA is a dependable result, as each deleted exon can be considered confirmation of the other deleted exon(s). If only one exon is deleted, and no heterozygous polymorphism is detected by sequencing (especially under the probe), then a second confirmatory method such as quantitative PCR (qPCR) is recommended. Other methods used for detection of large gene rearrangements include multiplex amplifiable probe hybridization (MAPH) and qPCR analysis.
Deletions in the 3’ region of the EPCAM gene can be detected using MLPA or gene-targeted aCGH. The same considerations apply to the hereditary colonic polyposis genes: APC, MUTYH, POLD1, and POLE.
Due to the pseudogene interference, the deletion of PMS2 is not reliably detected by NGS or MLPA.
Next-generation sequencing
The cost of sequencing has dropped rapidly in the past several years, and sequencing all genes implicated in colorectal cancer in a panel costs approximately the same as Sanger sequencing for a single or few genes. NGS can be used to sequence all 23 genes using target enrichment. The PMS2 gene cannot be sequenced using this technology due to the presence of pseudogenes (www.ucsc.edu). Target enrichment involves selection using a PCR-based method, such as highly multiplex PCR and digital PCR or in-solution hybridization-based methods. Following gene selection, NGS can be performed using short- or long-read technologies. NGS data analysis is complex and requires significant bioinformatics input. Annotation and variant classification require substantial effort due to the sizeable data quantities generated48 (see Fig. 2).
Irrespective of the target selection method used, all regions of interest (exons and flanking intron/exon boundaries) must have >20× coverage and average coverage of 100×.49 Regions not covered or sequenced adequately will dictate Sanger sequencing or orthogonal method to complete clinical testing. Also, technical limitations currently preclude detection of single-exon and multiexon deletions and duplications, and may require other methodologies. Recently, rare variants such as transposons/mobile elements have been reported.50 These may not be detected by routine Sanger or NGS based assays and need advanced bioinformatic scripts. It is important to confirm the laboratory’s methodology when ordering and reviewing the clinical report.
Compared to a sequential gene-by-gene testing approach, a disease-targeted NGS panel focused on the simultaneous analysis of the 23 CRC and polyposis risk genes is often a suitable cost-effective alternative while retaining maximum sensitivity. The coding exons with flanking intronic regions typically are used to define the region of interest (ROI) for analysis. For some genes where pathogenic variation has been described in the 5’ or 3’ UTR or promotor region, such as PTEN, these relevant regions are also included in the ROI. Targeted regions should minimally include coding exons with sufficient intronic coverage to allow analysis of positions -1_-16 and +1_+5 as well as other regions with reported pathogenic variants (e.g., splice sites of noncoding exons, deep intronic variants).51 The targeted genes and ROI regions will be captured by either RNA or DNA baits and sequenced on an NGS platform.
It is important to recognize technical limitations of the NGS technology—for example, interference of homologous sequences. The PMS2 and PTEN genes have highly homologous pseudogenes that may not be adequately captured or sequenced to allow for confidence in data quality. These genes may need to be tested separately using other methodologies, such as long-range PCR following by nested Sanger sequencing. Furthermore, NGS data have been used to detect exon-level deletions or duplications. The analytical sensitivity and specificity of small deletions or duplications (involving 1 or 2 exons) are, however, still low at approximately 80%. It may be necessary to confirm exonic deletions or duplication by a secondary assay, such as high-density microarray or qPCR.
RESULT INTERPRETATION
The following elements must be included in the clinical report.
Microsatellite instability by PCR
The panel of repeats used, as well as the results from each repeat, should be reported. MSI-H is reported if 40% or more of the repeats are unstable; MSI-stable is reported if no repeats are unstable, and MSI-L is reported if fewer than 40% of repeats are unstable. Only a high degree of microsatellite instability (MSI-H) is considered to be indicative of potential LS.
MMR protein defect detection by IHC
The results for antibodies to all four MMR proteins (MLH1, MSH2, MSH6, and PMS2) should be reported as normal or retained expression, abnormal or loss of expression, or uninterpretable. Uninterpretable refers to a lack of tumor staining without internal control positivity. Quantification of the strength of antibody staining is not recommended.
Germline variant testing for hereditary colon cancer or polyposis
The clinical report should provide genomic coordinate with genome build, gene name, reference transcript, zygosity, complementary DNA (cDNA) nomenclature, nucleotide change, nomenclature for the predicted or known protein impact when appropriate, and variant classification or clinical assertion.52 The associated disease(s) and inheritance pattern should be described. Use of Human Genome Variation Society nomenclature52 is recommended. Refer to the ACMG/AMP standards and guidelines for interpretation of sequence variants.48
Guidelines
NCCN guidelines for familial CRC and polyposis and general guidelines for clinical NGS sequencing assays have been published by the ACMG, CAP, National Committee for Clinical Laboratory Standards (CLSI), and the AMP.
References
Pearlman, R. et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 3, 464–471 (2017).
National Comprehensive Cancer Network. Clinical practice guidelines in oncology genetic/familial high-risk assessment: colorectal. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf (2020).
Seifert, B. A. et al. Determining the clinical validity of hereditary colon cancer and polyposis susceptibility genes using the Clinical Genome Resources Clinical Validity Framework. Genet. Med. 21, 1507–1516 (2019).
Goodenberger, M. & Lindor, N. M. Lynch syndrome and MYH-associated polyposis: review and testing strategy. J. Clin. Gastroenterol. 45, 488–500 (2011).
Markowitz, S. D. & Bertagnolli, M. M. Molecular origins of cancer: molecular basis of colorectal cancer. N. Engl. J. Med. 361, 2449–2460 (2009).
Vasen, H. F. A. Review article: the Lynch syndrome (hereditary nonpolyposis colorectal cancer). Aliment Pharmacol. Ther. 26, 113–126 (2007).
Umar, A. et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 96, 261–268 (2004).
Umar, A., Risinger, J. I., Hawk, E. T. & Barrett, J. C. Testing guidelines for hereditary nonpolyposis colorectal cancer. Nat. Rev. Cancer. 4, 153–158 (2004).
Win, A. K. et al. Determining the frequency of de novo germline mutations in DNA mismatch repair genes. J. Med. Genet. 48, 530–534 (2011).
Felton, K. E. A., Gilchrist, D. M. & Andrew, S. E. Constitutive deficiency in DNA mismatch repair: is it time for Lynch III? Clin. Genet. 71, 499–500 (2007).
Felton, K. E. A., Gilchrist, D. M. & Andrew, S. E. Constitutive deficiency in DNA mismatch repair. Clin. Genet. 71, 483–498 (2007).
Hegde, M. R. et al. A homozygous mutation in MSH6 causes Turcot syndrome. Clin. Cancer Res. 11, 4689–4693 (2005).
Hayward, B. E., De Vos, M., Sheridan, E. & Bonthron, D. T. PMS2 mutations in HNPCC. Clin. Genet. 66, 566–567 (2004).
Lynch, P. M. The hMSH2 and hMLH1 genes in hereditary nonpolyposis colorectal cancer. Surg. Oncol. Clin. N. Am. 18, 611–624 (2009).
Genuardi, M. et al. MLH1 and MSH2 constitutional mutations in colorectal cancer families not meeting the standard criteria for hereditary nonpolyposis colorectal cancer. Int. J. Cancer. 75, 835–839 (1998).
Giraldez, M. D. et al. MSH6 and MUTYH deficiency is a frequent event in early-onset colorectal cancer. Clin. Cancer Res. 16, 5402–5413 (2010).
Duraturo, F. et al. Association of low-risk MSH3 and MSH2 variant alleles with Lynch syndrome: probability of synergistic effects. Int. J. Cancer. 129, 1643–1650 (2011).
Nicolaides, N. C. et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 371, 75–80 (1994).
Talseth-Palmer, B. A., McPhillips, M., Groombridge, C., Spigelman, A. & Scott, R. J. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered. Cancer Clin. Pract. 8, 5 (2010).
Rumilla, K. et al. Frequency of deletions of EPCAM (TACSTD1) in MSH2-associated Lynch syndrome cases. J. Mol. Diagn. 13, 93–99 (2011).
Lynch, H. T., Lynch, J. F., Snyder, C. L. & Riegert-Johnson, D. EPCAM deletions, Lynch syndrome, and cancer risk. Lancet Oncol. 1, 5–6 (2011).
Morak, M. et al. Prevalence of CNV-neutral structural genomic rearrangements in MLH1, MSH2, and PMS2 not detectable in routine NGS diagnostics. Fam. Cancer. 19, 161–167 (2020).
Rhees, J., Arnold, M. & Boland, C. R. Inversion of exons 1-7 of the MSH2 gene is a frequent cause of unexplained Lynch syndrome in one local population. Fam. Cancer. 13, 219–225 (2014).
Liu, Q. et al. A cryptic paracentric inversion of MSH2 exons 2-6 causes Lynch syndrome. Carcinogenesis. 37, 10–17 (2016).
Yuan, Z. Q. et al. A missense mutation in both hMSH2 and APC in an Ashkenazi Jewish HNPCC kindred: implications for clinical screening. J. Med. Genet. 36, 790–793 (1999).
Barrow, E., Hill, J. & Evans, D. G. Cancer risk in Lynch syndrome. Fam. Cancer. 12, 229–240 (2013).
Perez-Carbonell, L. et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut. 61, 865–872 (2012).
Poulogiannis, G., Frayling, I. M. & Arends, M. J. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology. 56, 167–179 (2010).
Bartley, A. N., Luthra, R., Saraiya, D. S., Urbauer, D. L. & Broaddus, R. L. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev. Res. (Phila). 5, 320–327 (2012).
Kim, Y. H., Kakar, S., Cun, L., Deng, G. & Kim, Y. S. Distinct CpG island methylation profiles and BRAF mutation status in serrated and adenomatous colorectal polyps. Int. J. Cancer. 123, 2587–2593 (2008).
Deng, G. et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin. Cancer Res. 10, 191–195 (2004).
Loughrey, M. B. et al. Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary nonpolyposis colorectal cancer. Fam. Cancer. 6, 301–310 (2007).
Haraldsdottir, S. et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 147, 1308–1316.e1 (2014).
Bonadona, V. et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 305, 2304–2310 (2011).
Stoffel, E. M. & Chittenden, A. Genetic testing for hereditary colorectal cancer: challenges in identifying, counseling, and managing high-risk patients. Gastroenterology. 139, 1436–1441 (2010).
Gismondi, V. et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int. J. Cancer. 109, 680–684 (2004).
Poulsen, M. L. M. & Bisgaard, M. L. MUTYH associated polyposis (MAP). Curr. Genomics. 9, 420–435 (2008).
Strande, N. T. et al. Evaluatimg the clinical validity of gene–disease associations: an evidence-based framework developed by the Clinical Genome Resource. Am. J. Hum. Genet. 100, 895–906 (2007).
Bean, L. J. H. et al. Diagnostic gene sequencing panels: from design to report-a technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 22, 453–461 (2020).
Yurgelun, M. B. et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J. Clin. Oncol. 35, 1086–1095 (2017).
Stoffel, E. M. et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology. 154, 897–905.e1 (2018).
Manavis, J., Gilham, P., Davies, R. & Ruszkiewicz, A. The immunohistochemical detection of mismatch repair gene proteins (MLH1, MSH2, MSH6, and PMS2): practical aspects in antigen retrieval and biotin blocking protocols. Appl. Immunohistochem. Mol. Morphol. 11, 73–77 (2003).
Pearlman, R. et al. Two-stain immunohistochemical screening for Lynch syndrome in colorectal cancer may fail to detect mismatch repair deficiency. Mod. Pathol. 31, 1891–1900 (2018).
Hampel, H. et al. Assessment of tumor sequencing as a replacement for Lynch syndrome screening and current molecular tests for patients with colorectal cancer. JAMA Oncol. 4, 806–813 (2018).
Ogino, S. et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J. Mol. Diagn. 8, 209–217 (2006).
Fukushima, T. et al. Promoter hypermethylation of mismatch repair gene hMLH1 predicts the clinical response of malignant astrocytomas to nitrosourea. Clin. Cancer Res. 11, 1539–1544 (2005).
Hendriks, Y. M. C. et al. Heterozygous mutations in PMS2 cause hereditary nonpolyposis colorectal carcinoma (Lynch syndrome). Gastroenterology. 130, 312–322 (2006).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Santani, A. et al. Designing and implementing NGS tests for inherited disorders: a practical framework with step-by-step guidance for clinical laboratories. J. Mol. Diagn. 21, 369–374 (2019).
Solassol, J. et al. Alu element insertion in the MLH1 exon 6 coding sequence as a mutation predisposing to Lynch syndrome. Hum. Mutat. 40, 716–720 (2019).
Rehder, C. et al. Next-generation sequencing for constitutional variants in the clinical laboratory, 2021 revision: a technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. https://doi.org/10.1038/s41436-021-01139-4 (2021).
Human Genome Variation Society. Sequence variant nomenclature, version 19.01. https://varnomen.hgvs.org/ (2020).
Author information
Authors and Affiliations
Consortia
Ethics declarations
Competing interests
R.M., P.K., R.P.G., A.G., M.F. and M.H. are employed by fee-for-service laboratories performing NGS. The other authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer
This technical standard is designed primarily as an educational resource for clinical laboratory geneticists to help them provide quality clinical laboratory genetic services. Adherence to this technical standard is voluntary and does not necessarily assure a successful medical outcome. This technical standard should not be considered inclusive of all proper procedures and tests or exclusive of other procedures and tests that are reasonably directed to obtaining the same results. In determining the propriety of any specific procedure or test, the clinical laboratory geneticist should apply his or her own professional judgment to the specific circumstances presented by the individual patient or specimen.
Clinical laboratory geneticists are encouraged to document in the patient’s record the rationale for the use of a particular procedure or test, whether or not it is in conformance with this technical standard. They also are advised to take notice of the date any particular technical standard was adopted, and to consider other relevant medical and scientific information that becomes available after that date. It would also be prudent to consider whether intellectual property interests may restrict the performance of certain tests and other procedures.
Rights and permissions
About this article
Cite this article
Mao, R., Krautscheid, P., Graham, R.P. et al. Genetic testing for inherited colorectal cancer and polyposis, 2021 revision: a technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet Med 23, 1807–1817 (2021). https://doi.org/10.1038/s41436-021-01207-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-021-01207-9
This article is cited by
-
Detection of germline variants with pathogenic potential in 48 patients with familial colorectal cancer by using whole exome sequencing
BMC Medical Genomics (2023)
-
Diagnosis of patients with Lynch syndrome lacking the Amsterdam II or Bethesda criteria
Hereditary Cancer in Clinical Practice (2023)
-
Genotype–Phenotype Correlations in Autosomal Dominant and Recessive APC Mutation-Negative Colorectal Adenomatous Polyposis
Digestive Diseases and Sciences (2023)
-
Prevalence and genetic spectrum associated with hereditary colorectal cancer syndromes, the need to improve cancer risk awareness, and family cascade testing in Vietnam
Familial Cancer (2023)
-
Colorectal cancer incidences in Lynch syndrome: a comparison of results from the prospective lynch syndrome database and the international mismatch repair consortium
Hereditary Cancer in Clinical Practice (2022)