Abstract
Purpose
The aim was to assess lifetime risk for hospitalization in individuals with neurofibromatosis 1 (NF1).
Methods
The 2467 individuals discharged with a diagnosis indicating NF1 or followed in a clinical center for NF1 were matched to 20,132 general population comparisons. Based on diagnoses in 12 main diagnostic groups and 146 subcategories, we calculated rate ratios (RRs), absolute excess risks (AERs), and hazard ratios for hospitalizations.
Results
The RR for any first hospitalization among individuals with NF1 was 2.3 (95% confidence interval 2.2–2.5). A high AER was seen for all 12 main diagnostic groups, dominated by disorders of the nervous system (14.5% of all AERs), benign (13.6%) and malignant neoplasms (13.4%), and disorders of the digestive (10.5%) and respiratory systems (10.3%). Neoplasms, nerve and peripheral ganglia disease, pneumonia, epilepsy, bone and joint disorders, and intestinal infections were major contributors to the excess disease burden caused by NF1. Individuals with NF1 had more hospitalizations and spent more days in hospital than the comparisons. The increased risk for any hospitalization was observed for both children and adults, with or without an associated cancer.
Conclusion
NF1 causes an overall greater likelihood of hospitalization, with frequent and longer hospitalizations involving all organ systems throughout life.
Similar content being viewed by others
INTRODUCTION
Neurofibromatosis 1 (NF1 [MIM 162200]) is a common Mendelian disorder, affecting 1 in 2500–3000 individuals worldwide.1,2 It has autosomal dominant inheritance, but many sporadic cases result from de novo NF1 variants.3 The diagnosis usually derives from clinical elements, including café-au-lait skin macules, axillary and inguinal freckling, iris Lisch nodules, neurofibromas, and dysplasia of the long bones, vertebrae, and cranium.4 The frequency and nature of these and other manifestations vary widely, even for the same variant,5 making it difficult to predict the phenotype or severity at initial diagnosis.6
Individuals with NF1 have an increased mortality compared with the general population,7 with a decreased life expectancy of approximately 15 years mainly due to deaths from malignancies.8,9 The increased cancer risk has been estimated in various studies. Most recently, a large Finnish register-based study delineated the lifetime risk for cancer in 1404 individuals with NF1. The risk for cancer was highly increased in childhood, adolescence, and young adulthood, but the excess risk continued throughout life. Exceptionally high risks were found for NF1-related cancers, including malignant peripheral nerve sheath tumors and cancer in the autonomic nervous system, but increased risks for breast cancer and cancer of the digestive organs and adrenal glands were also noticed.10 Other clinical problems have also been reported in individuals with NF1, including fractures,11 cardiovascular compromises,12 neurocognitive deficits,13 and benign tumors of the nervous system.14 However, the full range of somatic morbidities seen in a single nation over entire life spans is still unknown. We therefore conducted a population-based retrospective cohort study of the risk for hospitalization, utilizing standardized diagnostic terminology, for 2467 individuals with NF1 in Denmark, and 20,132 general comparison subjects without NF1. The purpose was to determine the lifetime risks for somatic hospitalizations among individuals with NF1 using the unique Danish nationwide registries that provide long-term and virtually complete follow-up of the Danish population. Due to the high risk of cancer in individuals with NF1 and the well-known risk of diseases affecting almost all organs and body systems following cancer treatment even without NF1,15,16 we stratified cohort members into those with or without an associated cancer.
MATERIALS AND METHODS
The large register-based NF1 cohort consisted of all 2576 individuals who have been discharged from a hospital in Denmark with the diagnosis of NF according to the International Classification of Diseases version 8 (ICD-8) 743.49 or ICD-10 Q85.0 during the period 1977–2013 and/or followed in one of two centers for rare diseases in Denmark (see flow chart in Fig. S1). All had to be alive on or after 1 January 1968, when all Danish inhabitants were assigned a unique personal identification number, allowing accurate linkage among registries. The Danish National Patient Register (DNPR), established in 1977, includes data on all hospital admissions for nonpsychiatric diseases. Registration is mandatory and is provided by the patient’s treating physician. Each admission initiates a record that includes dates of admission and discharge, a primary discharge diagnosis, and up to 19 supplementary diagnoses coded according to ICD-8 until 1993 and ICD-10 thereafter.17
It is not possible to differentiate between the two subtypes of neurofibromatosis, NF1 and NF2, in the DNPR. Thus, to only include patients with NF1, we excluded patients with symptoms of NF2,18 including (1) uni- or bilateral acusticus neurinoma or other cranial nerves affected but n. opticus, (2) multiple meningiomas, (3) at least one meningioma and a discharge history with cardinal clinical features strongly indicative of NF2, and (4) optic nerve meningioma according to the original notification forms to the Danish Cancer Registry and the discharge history of the respective patients obtained from the DNPR. Based on these criteria, 59 patients were excluded from the large registry-based patient cohort with tumors or tumor combinations and a discharge history compatible with NF2.
The NF1 Registry cohort consisted of 637 individuals with NF1 followed between 1995 and 2013 at the National Centers of Rare Diseases at Copenhagen University Hospital, Rigshospitalet, and Aarhus University Hospital. This cohort included all known individuals in Denmark who fulfill the US National Institutes of Health (NIH) criteria for NF119 or had the diagnosis confirmed by molecular genetic testing with findings of a variant or deletion in the NF1 gene. All patients with NF1 are recommended to be followed in one of the two centers, independent of their age, income, social class, and disease severity.
For each patient, we randomly selected ten general population comparison subjects from the Danish Civil Registration System matched to NF1 individuals on sex and date of birth. They had to be alive on 1 January 1968, or be born thereafter and without registration of NF1 diagnosis in either the DNPR or the NF1 Registry on the date their matched patient was diagnosed with NF1 (n = 25,170). Information on vital status and migrations was ascertained from the Danish Civil Registration System.
Exclusion of individuals with NF1 and population comparisons
We excluded 37 individuals with NF1 who had no date of diagnosis in the NF1 Registry or a changed discharged diagnosis and 13 patients who did not live in Denmark when they were diagnosed with NF1. We also excluded 453 comparisons with no matched case, 1187 comparisons who died before their matched case was diagnosed with NF1, as well as 30 doublet comparisons. Finally, we excluded 3368 comparisons who did not live in Denmark when their matched case was diagnosed with NF1. After these exclusions, the main register-based study cohort consisted of 2467 individuals with NF1, including 614 individuals from the clinical NF1 Registry (of whom 76 individuals had never been discharged from the hospital with a NF1 diagnosis). The comparison cohort included 20,132 population comparisons.
Information on inpatient hospital admission and cancer
The DNPR provided a full hospital history for all inpatient hospitalizations from 1977 to 2016. The primary discharge diagnoses were grouped into 12 main diagnostic groups, which were further divided into 146 specific categories utilizing ICD-8 and ICD-10 codes (see Table S1 for a complete list of all included diagnostic groups and diagnoses). All main groups were included, except for injuries and violence, and those reflecting symptoms alone or otherwise incompletely defined diagnostic categories. Also excluded were mental disorders as well as childbirth and pregnancy-related hospitalizations that will be addressed in two separate studies. To identify individuals with cancer, the cohort members were linked to the Danish Cancer Registry, which has information on cancer diagnoses nationwide since 1943. Cancers were classified according to the ICD-7 for 1943–1977, ICD for Oncology for 1978–2003, and ICD-10 thereafter.20
Statistical analysis
Follow-up for the specified hospital-based diagnoses started on date of first discharge diagnosis of NF1 from the DNPR after 1 January 1977, or date of diagnosis in the NF1 Registry, whichever came first. The matched comparisons were followed from the same date. Follow-up ended at date of death, emigration, date of first discharge diagnosis of NF1 (only comparisons), or end of study on 31 December 2016, whichever occurred first. We estimated hospitalization rates, rate ratios (RRs), and absolute excess risks (AERs) for any first hospitalization in the follow-up period between 1977 and 2016 by sex and attained age (0–9, 10–14, 15–19, 20–29, 30–39, 40–49, 50–59, ≥60 years), in one of 12 main diagnostic groups and for each of 146 diagnostic categories. We also used Cox proportional hazards models with age as the underlying time scale to estimate the hazard ratio (HR) both for any first hospitalization during follow-up and for each first hospitalization in the main diagnostic groups. Cancer status was modeled as a time-varying covariate, and effect modification of cancer was included as an interaction term. The time scale was split into age intervals: eight intervals as defined above for any first hospitalization during follow-up and four intervals for the main diagnostic groups (0–14, 15–29, 30–49, ≥50 years). All models were stratified by sex and birth decade. An interaction term for sex was included to investigate any sex differences. The total disease burden for both the first hospitalization during follow-up and readmissions was estimated as the expected number of any hospitalizations over time (age) for an individual using the method of mean cumulative count.21 Based on all hospitalizations, we calculated the total number of bed days spent in hospital by sex and cancer status for each hospitalization and in each main diagnostic group, expressed as bed day ratios (BDRs) with 95% confidence intervals (95% CIs) from negative binomial models adjusted for year of birth. Finally, a sensitivity analysis was conducted calculating the RR and HR for any first hospitalization during follow-up stratified by sex and age. This analysis included the 614 patients in the NF1 Registry cohort for whom their NF1 diagnosis is certain and their 5046 matched comparisons. All risk estimates are presented with 95% CIs. If less than four events were observed in a group, <4 was reported due to reporting restrictions from Statistics Denmark. The analyses were performed in R22 with packages “etm” (version 0.6.2), “survival” (2.41.3), “ggplot2” (2.2.1), and “fmsb” (0.6.1).
The study was approved by the Danish Protection Agency (Record 2014–41–2935) and the Human Research Protection Office (HRPO) (A:18370.i). The study group welcomes collaboration with other researchers using our registry data. Study protocols can be planned in collaboration with us, and the study material can be analyzed accordingly at the server of Statistics Denmark, where all data are stored.
RESULTS
The 2467 individuals with NF1 and 20,132 population comparisons had 10,276 and 52,609 hospitalizations during a median follow-up of 14.8 and 16.5 years (range 0–40.8), respectively. Additional main characteristics of the cohort members are seen in Table 1.
The overall rate ratio (RR) for any first hospitalization was 2.3 (95% CI 2.2–2.5) for individuals with NF1. The RR varied from 1.9 (95% CI 1.6–2.2) in the age group 20–29 years to 3.9 (95% CI 3.5–4.4) for children below the age of 10 (Table 2). For individuals with a verified diagnosis of NF1 in the NF1 Registry cohort, the overall RR was 2.6 (2.3–2.9) (data not shown).
Based on hospitalization rates among individuals with NF1 and population comparisons, the absolute excess risk (AER expressed per 10,000 person-years) of NF1 individuals for a new admission to hospital was 543 per 10,000 person-years. That is, for each additional year of follow-up, 5 of 100 individuals with NF1 were hospitalized with a new excess diagnosis. Benign neoplasms (AER: 75 per 10,000 person-years), malignant neoplasms (AER: 74), disorders of the nervous system and sense organs (AER: 80), and disorders of the digestive (AER: 58) and respiratory systems (AER: 57) constituted 62.3% (344/552) of all excess hospitalizations (Table 2). Especially, cancer of the eye, brain, and other parts of the central nervous system (CNS) (AER: 39) and mesothelium (AER: 19), disorders of nerves and peripheral ganglia (AER: 30), pneumonia (AER: 23), epilepsy (AER: 22), osteomyelitis and other disorders of bones and joints (AER: 21), and benign tumors of peripheral and autonomic nervous system (AER: 20) contributed to the excess risk. Other common clinical problems with an increased risk involved the digestive, respiratory, urinary, and circulatory systems. These diagnoses included, for example, hernia of the abdominal cavity (RR 1.7, 95% CI 1.3–2.0), diseases of the teeth and supporting structures (RR 4.3, 95% CI 3.0–6.0), respiratory failure (RR 2.2, 95% CI 1.7–2.9), urinary infections (RR 1.8, 95% CI 1.5–2.3), cerebrovascular disease (RR 1.6 95% CI, 1.3–1.9), and venous and lymphatic problems (RR 1.6, 95% CI 1.3–2.1). Only diabetes mellitus type 1 was significantly decreased in individuals with NF1 (RR 0.4, 95% CI 0.2–0.97) (see a complete list of all risk estimates in Table S2).
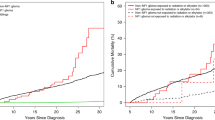
Over various age groups, more NF1 individuals were hospitalized for any diagnosis in each of the 12 diagnostic groups compared with the population comparisons (Fig. 1a). When we included first hospitalizations for any of the 146 selected diagnoses during follow-up, the mean number of hospitalizations was approximately twice as high among individuals with NF1 relative to population comparisons up to the age of 70 years (Fig. 1b). The mean numbers of all hospitalizations for individuals with NF1 and population comparisons under the age of 20 years were approximately 4 and 1, respectively. These increased to approximately 12 and 4.5 by the age of 60 years (Fig. 1c).
(a) Incidence of first hospitalizations in the 12 main diagnostic groups for individuals with neurofibromatosis 1 (NF1) and for population comparison subjects, by age. Mean frequency of first (b) and all hospitalizations (c) with 95% confidence interval for any of the 146 selected disorders for individuals with NF1 (red line) and population comparisons (blue line), by age.
For females with NF1 (excluding pregnancy and delivery hospitalizations), the overall BDRs were 10.1 (95% CI 8.8–11.7) for those with and 2.4 (95% CI 2.1–2.6) for those without a previous cancer diagnosis, with similar BDRs in males with NF1 being 9.8 (95% CI 7.7–12.4) and 2.3 (95% CI 1.8–2.8) (Fig. 2). The highest BDRs were seen for disorders of the nervous system, benign tumors, and infectious diseases among females with NF1 and cancer. For males with cancer, benign tumors and diseases of blood and nervous system led to a high number of days spent in hospital.
Number of bed days (BDs) in hospital and associated bed day ratios (BDRs) with 95% confidence intervals (CIs) for hospitalizations for any disease (except cancer) and in each of the 11 main diagnostic groups in individuals with neurofibromatosis 1 (NF1) and population comparisons (referent), by sex and previous cancer diagnosis (circle, yes; triangles, no). Note: x-axis is logarithmic.
Individuals with NF1 and cancer experienced the highest risks, but the HRs for any hospitalization were also increased among those without a prior cancer diagnosis (Fig. 3). The risks remained increased across all age groups. The results from the analysis only including patients from the clinical NF1 Registry showed a similar pattern with highest HRs in childhood (data not shown).
Hazard ratios (HRs) with 95% confidence intervals (CIs) for first hospitalization in individuals with neurofibromatosis 1 (NF1) and population comparisons (referent), by sex, age, and previous cancer diagnosis (circles, yes; triangles, no). *Significant (p value <0.05) for effect modification of cancer diagnosis.
For the results on the 11 main diagnostics groups (except cancer), especially high HRs were seen in children. The HRs for infectious and parasitic diseases, endocrine diseases, diseases of the blood, as well as disorders of the nervous and circulatory system were more than three times higher in children with NF1, irrespective of a previous cancer diagnosis, than in children in the comparison group (Fig. S2A–C). The HRs continued to be increased throughout life. Finally, we showed a higher cumulative incidence for any hospitalization in any of the main diagnostic groups as well an increased mortality among individuals with NF1 than in the population comparisons, irrespective of cancer status (Fig. S2A–C).
DISCUSSION
This population-based study gives the most comprehensive overview to date of the risk of hospitalization for somatic diagnoses among individuals with NF1 over the life span, including serious health problems that first cause hospitalization in middle age or senescence. Individuals with NF1 were hospitalized twice as much for any clinical diagnosis as the population comparisons and this risk persisted throughout life, with 235 to 1258 excess diagnosis-specific hospitalizations per 10,000 individuals with NF1 per year; i.e., hospitalizations for clinical problems most likely attributable to NF1. Excess hospitalizations were seen for all main diagnostic groups, verifying that NF1 and its associated clinical problems consistently involve all 12 diagnostic categories. Besides spending more days in hospital, NF1 individuals had also 2.5 times as many hospitalizations by age 60 years as the population comparisons. These findings re-emphasize the frequent and variable clinical manifestations in NF1, consistent with prior investigations, but quantified here for the first time.
The large Finnish register-based study of cancer risk in individuals with NF1 reported that the estimated lifetime probability of cancer was 59.6% in individuals with NF1, compared with 30.8% in the general Finnish population.10 As expected, we found that the risks for study-eligible health problems and extra days spent in hospitals were highest for those with both NF1 and cancer. However, NF1 individuals without any cancer diagnosis also experienced a higher disease burden than the general population. Not surprisingly, benign tumors contributed mostly to the excess risk in individuals with NF1 and additional bed days spent in hospital. Although neurofibromas, the benign peripheral nerve sheath tumors that form in association with spinal, peripheral, or cranial nerves, are an important feature of NF1, the disorder ranges far beyond neurofibromas. To understand the clinical burden of NF1, it is important to focus not only on the manifestations of NF1 (e.g., skeletal dysplasia), but also on the consequences of the features (e.g., pain because of skeletal dysplasia) and the complications that NF1 individuals experience from these consequences (e.g., disfigurement or spinal cord compression).23 Our results document the widespread somatic consequences and complications of NF1, which can have extensive socioeconomic consequences, including challenges with educational attainment and employment, and quality of life.
Disorders of the nervous system and sense organs also contributed substantially to the excess hospitalizations and increased risks, especially diseases of nerves and peripheral ganglia (RR 4.5) and epilepsy (RR 5.5). Other studies have found a prevalence of epilepsy up to 14% in individuals with NF1.24 To our knowledge, only one study has reported a risk estimate for epilepsy in individuals with NF1. Madubata et al. showed an odds ratio (OR) for health insurance claims for epilepsy of 7.3 (95% CI 6.4–8.3) in a large study of 8579 individuals with NF1 identified in a claim database of the privately insured US population.25 The increased prevalence of seizures in NF1 has been related to intracranial tumors, including optic pathway gliomas,26 which are some of the most common tumors in children with NF1.
Previous studies have also shown other neurologic complications of NF1, including pain from scoliosis and spinal neurofibromas as well as hydrocephalus and headache from CNS tumors.27,28,29,30 Besides the location of a tumor in the nervous system, the subsequent cancer management might also cause other neurologic problems requiring inpatient care31 as seen in the much higher risk for being hospitalized with a disorder of the nervous system and the number of days spent in hospital among those individuals with both NF1 and cancer.
High AERs and increased RRs were also seen for several digestive disorders. However, only a few studies have explored digestive problems related to NF1.32,33 A Danish clinical study examined gastrointestinal symptoms in 175 individuals with NF1 identified in the NF1 Registry. The overall likelihood of fulfilling the diagnostic criteria for functional constipation, irritable bowel syndrome, or functional dyspepsia was higher among patients than in their unaffected relatives.33 Focus on gastrointestinal problems, including the increased risk for disorders of the teeth as outlined in our study, is needed. Early detection and diagnosis is important given the risk of the complications, including malignancies. The high risks for disorders of the respiratory system, including pneumonia and acute respiratory failures, have not previously been reported. As these disorders can be life threatening, further studies are warranted to elucidate the underlying causes.
We noted an increased risk for both NF1-specific cancer (cancer of the brain and CNS as well as mesothelium and connective tissue) and cancer that might be related to NF1, including gastrointestinal (RR 1.4, 95% CI 1.04–1.8) and breast cancer (RR 1.6, 95% CI 1.2–2.2). The RR for breast cancer was lower than the overall risk reported in a Finnish study on breast cancer in NF1 (standardized incidence ratio [SIR] 2.82, 95% CI 1.92–4.00). In this study, females younger than 40 years had the highest risk (SIR 14.25, 95% CI 6.51–27.04), while the risk in females ≥60 years was increased (SIR 1.86, 95% CI 0.80–3.66), but did not reach statistical significance.34 The lower estimate observed in our study is probably due to an older patient group included in our NF1 cohort. The increased risks for a variety of cancers reported here confirm the importance of follow-up of patients with NF1 in highly specialized centers.
In our study, most events within the circulatory group were seen for cerebrovascular disease with a RR of 1.6 (95% CI 1.3–1.9). An increased risk has also been reported in a prior population-based case–control study among 601 individuals with NF1 (OR 1.2, 95% CI 1.1–1.3).35 We also delineated increased risks for other cardiovascular compromises, including venous and lymphatic disease and hypertensive disorders. Cardiovascular disease can be seen as a complication of NF vasculopathies and is a serious threat with early mortality.9
Interestingly, we observed that type 1 diabetes seemed to be less common in individuals with NF1 than in the general population. Lower fasting blood glucose levels have been seen in individuals with NF1 compared with non-NF1 controls matched by sex, age and body mass index (BMI),36 as well as a low mortality due to diabetes mellitus.9 Further, individuals with NF1 have been found to have a lower risk for having health insurance claims for diabetes (OR 0.4, 95% CI 0.3–0.4) compared with a healthy control group.25 Finally, a study reported an increased insulin sensitivity in 40 individuals with NF1, which may be associated with the lower occurrence of type 2 diabetes mellitus previously reported in this group of patients.37 The causes of these observations in NF1 are not well established, and further studies are required to elucidate the association.
Strengths and limitations
A major strength of our study is the nationwide, population-based design, including a large number of individuals with NF1 and a population-based comparison cohort for whom we have virtually complete follow-up. Further, our study was based on diagnostic information reported by medical professionals instead of self-reported outcomes, increasing the validity of the outcomes. We excluded individuals with clinical and anatomic components of NF2 in the register, but we were not able to identify those only hospitalized before the establishment of the DNPR. Although we did not review medical records of all included individuals with NF1, we conducted a subanalysis including only those with NF1 who are followed in one of two national centers for rare disease and for whom the NF1 diagnosis is certain. The overall risk estimate for any first hospitalization during follow-up increased only slightly from 2.3 (95% CI 2.2–2.5) to 2.6 (95% CI 2.3–2.9) in this analysis, suggesting that their overall hospitalization risk is similar.
To be included in the register-based cohort, individuals were hospitalized with or for NF1. Individuals with NF1 who were less affected by their disease and therefore not hospitalized or followed in the rare disease clinics were not included in our study, so our results cannot be generalized to those with minimal medical NF1 complications. Thus, if the patient inclusion is biased toward a more severe NF1 phenotype or those with classical symptoms of NF1, the associations reported in this study may overestimate the true hospitalization risk in individuals with NF1. Further, the DPNR was established in 1977, and thus for those individuals older than 36 years, a potential gap of hospitalizations exists between birth and start of the register; however, this gap applies to both individuals with NF1 and the comparisons. This means that disorders originally detected before the start of the hospital register might be registered as incident events at a later hospitalization, and thus age at first event will be shifted toward an older age. Prior hospitalizations might be identified in medical records. As this study aimed at describing the overall disease burden in individuals with NF1, it was not possible to search all records for first hospitalization for all potential disorders in both NF1 patients and the comparison cohort.
We cannot exclude the possibility that our results were influenced by closer medical surveillance of patients with NF1 than of population comparison subjects. Surveillance bias would preferentially affect less well‐defined disorders such as hypertension, which will not always cause hospitalization. Finally, the delayed inclusion of individuals with NF1 might have caused some degree of selection bias, favoring those individuals with NF1 who have less tumor burden and better survival rates.
Conclusion
In conclusion, our study showed that individuals with NF1 have frequent clinical problems that persist and accumulate throughout life and require longer and more frequent hospitalizations. This fact and the accompanying quantification improve our understanding of this complex disease. As the consequences of living with this disorder can influence academic performance, education, employment, as well as quality of life, lifelong follow-up in specialized NF1 clinics with the experts to address the pleiotropic manifestations of the disease is important. Additional research is needed focusing on targeted interventions to include patient counseling, optimal follow-up, and support that address the findings outlined and emphasized here. As our large register-based cohort mainly included patients who have been hospitalized with NF, the true risks for the different disorders might be overestimated in our study and limit generalization outside the inpatient population. Thus, further studies of clinical complications in individuals with NF1 are warranted addressing the limitations described in this paper.
References
Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26:704–711.
Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005;141:71–74.
Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–843.
Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575–578.
Riccardi VM, Lewis RA. Penetrance of von Recklinghausen neurofibromatosis: a distinction between predecessors and descendants. Am J Hum Genet. 1988;42:284–289.
Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–351.
Sorensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med. 1986;314:1010–1015.
Zoller M, Rembeck B, Akesson HO, Angervall L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1. A twelve-year follow-up of an epidemiological study in Goteborg, Sweden. Acta Derm Venereol. 1995;75:136–140.
Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68:1110–1118.
Uusitalo E, Rantanen M, Kallionpaa RA, et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016;34:1978–1986.
Heerva E, Koffert A, Jokinen E, et al. A controlled register-based study of 460 neurofibromatosis 1 patients: increased fracture risk in children and adults over 41 years of age. J Bone Miner Res. 2012;27:2333–2337.
Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet Med. 2002;4:105–111.
Rosser TL, Packer RJ. Neurocognitive dysfunction in children with neurofibromatosis type 1. Curr Neurol Neurosci Rep. 2003;3:129–136.
Seminog OO, Goldacre MJ. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 2013;108:193–198.
de Fine Licht S, Rugbjerg K, Gudmundsdottir T, et al. Long-term inpatient disease burden in the Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study: a cohort study of 21,297 childhood cancer survivors. PLoS Med. 2017;14:e1002296.
Stricker CT, Jacobs LA. Physical late effects in adult cancer survivors. Oncology (Williston Park). Nurse Ed. 2008;22(8 Suppl):33–41.
Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33.
Ferner RE. The neurofibromatoses. Pract Neurol. 2010;10:82–93.
National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, Md., USA, July 13–15, 1987. Neurofibromatosis. 1988;1:172–8
Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45.
Hengelbrock J, Gillhaus J, Kloss S, Leverkus F. Safety data from randomized controlled trials: applying models for recurrent events. Pharm Stat. 2016;15:315–323.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017.
Riccardi VM. Neurofibromatosis type 1 is a disorder of dysplasia: the importance of distinguishing features, consequences, and complications. Birth Defects Res A Clin Mol Teratol. 2010;88:9–14.
Pecoraro A, Arehart E, Gallentine W, et al. Epilepsy in neurofibromatosis type. Epilepsy Behav. 2017;73:137–141.
Madubata CC, Olsen MA, Stwalley DL, Gutmann DH, Johnson KJ. Neurofibromatosis type 1 and chronic neurological conditions in the United States: an administrative claims analysis. Genet Med. 2015;17:36–42.
Ostendorf AP, Gutmann DH, Weisenberg JL. Epilepsy in individuals with neurofibromatosis type 1. Epilepsia. 2013;54:1810–1814.
Creange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122:473–481.
Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. A clinical and population study in south-east Wales. Brain. 1988;111:1355–1381.
Friedman JM, Birch PH. Type 1 neurofibromatosis: a descriptive analysis of the disorder in 1,728 patients. Am J Med Genet. 1997;70:138–143.
Riccardi VM. Neurofibromatosis: phenotype, natural history, and pathogenesis. 2nd ed. Baltimore, MD: Johns Hopkins University Press; 1992.
Kenborg L, Winther JF, Linnet KM, et al. Neurologic disorders in 4858 survivors of central nervous system tumors in childhood-an Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study. Neuro Oncol. 2019;21:125–136.
Basile U, Cavallaro G, Polistena A, et al. Gastrointestinal and retroperitoneal manifestations of type 1 neurofibromatosis. J Gastrointest Surg. 2010;14:186–194.
Ejerskov C, Krogh K, Ostergaard JR, Fassov JL, Haagerup A. Constipation in adults with neurofibromatosis type 1. Orphanet J Rare Dis. 2017;12:139.
Uusitalo E, Kallionpää RA, Kurki S, et al. Breast cancer in neurofibromatosis type 1: overrepresentation of unfavourable prognostic factors. Br J Cancer. 2017;116:211–217.
Terry AR, Jordan JT, Schwamm L, Plotkin SR. Increased risk of cerebrovascular disease among patients with neurofibromatosis type 1: population-based approach. Stroke. 2016;47:60–65.
Martins AS, Jansen AK, Rodrigues LOC, et al. Lower fasting blood glucose in neurofibromatosis type 1. Endocr Connect. 2016;5:28–33.
Martins AS, Jansen AK, Rodrigues LOC, et al. Increased insulin sensitivity in individuals with neurofibromatosis type 1. Arch Endocrinol Metab. 2018;62:41–46.
Acknowledgements
The study was supported by a grant from US Army Medical Research and Materiel Command (award number W81XWH-14–1–0054).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kenborg, L., Duun-Henriksen, A.K., Dalton, S.O. et al. Multisystem burden of neurofibromatosis 1 in Denmark: registry- and population-based rates of hospitalizations over the life span. Genet Med 22, 1069–1078 (2020). https://doi.org/10.1038/s41436-020-0769-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0769-6
Keywords
This article is cited by
-
The contribution of morbidity and unemployment for the reduced labor market participation of individuals with neurofibromatosis 1 in Finland
European Journal of Human Genetics (2024)
-
Burden of adult neurofibromatosis 1 questionnaire: translation and psychometric properties of the Persian version
Orphanet Journal of Rare Diseases (2023)
-
Employment, occupation, and income in adults with neurofibromatosis 1 in Denmark: a population- and register-based cohort study
Orphanet Journal of Rare Diseases (2023)
-
Clinical Characteristics and Management of Children and Adults with Neurofibromatosis Type 1 and Plexiform Neurofibromas in Denmark: A Nationwide Study
Oncology and Therapy (2023)
-
School performance of children with neurofibromatosis 1: a nationwide population-based study
European Journal of Human Genetics (2022)