Abstract
Purpose
To determine impact of risk-reducing hysterectomy and bilateral salpingo-oophorectomy (BSO) on gynecological cancer incidence and death in heterozygotes of pathogenic MMR (path_MMR) variants.
Methods
The Prospective Lynch Syndrome Database was used to investigate the effects of gynecological risk-reducing surgery (RRS) at different ages.
Results
Risk-reducing hysterectomy at 25 years of age prevents endometrial cancer before 50 years in 15%, 18%, 13%, and 0% of path_MLH1, path_MSH2, path_MSH6, and path_PMS2 heterozygotes and death in 2%, 2%, 1%, and 0%, respectively. Risk-reducing BSO at 25 years of age prevents ovarian cancer before 50 years in 6%, 11%, 2%, and 0% and death in 1%, 2%, 0%, and 0%, respectively. Risk-reducing hysterectomy at 40 years prevents endometrial cancer by 50 years in 13%, 16%, 11%, and 0% and death in 1%, 2%, 1%, and 0%, respectively. BSO at 40 years prevents ovarian cancer before 50 years in 4%, 8%, 0%, and 0%, and death in 1%, 1%, 0%, and 0%, respectively.
Conclusion
Little benefit is gained by performing RRS before 40 years of age and premenopausal BSO in path_MSH6 and path_PMS2 heterozygotes has no measurable benefit for mortality. These findings may aid decision making for women with LS who are considering RRS.
Similar content being viewed by others
INTRODUCTION
Lynch syndrome (LS) is a common hereditary cancer predisposition syndrome, present in an estimated 1 in 300 individuals, based on prevalence of the underlying genetic abnormalities in the general population. LS is caused by pathogenic variants in one of four DNA mismatch repair (MMR) genes (path_MMR): path_MLH1, path_MSH2, path_MSH6, and path_PMS2, each of which result in different risks for cancers, including colorectal, endometrial, ovarian, stomach, small bowel, bile duct, pancreas, urinary tract, brain, and prostate cancer.1,2,3,4,5 In women with LS, gynecological cancers are as common as gastrointestinal cancers. Until recently, clinical guidelines were similar for heterozygotes of all path_MMR genetic variants, endometrial cancer prognosis was assumed to be similar in heterozygotes and MMR variant-negative individuals, and the prognosis for ovarian cancer was assumed to be similar to ovarian cancer in path_BRCA1 heterozygotes. The recent Manchester International Consensus Group publication6 described the risk for, and survival after, gynecological cancers in LS by genotype, as initially reported by the Prospective Lynch Syndrome Database (PLSD).1,2,3,4,7 Later, the PLSD reported findings in an additional independent cohort of path_MMR heterozygotes that validated the results from its original cohort and allowed merger of both cohorts to obtain more precise risk estimates and calculation of 5-year and 10-year crude survival after cancer.2
Risk-reducing surgery (RRS) including total hysterectomy and bilateral salpingo-oophorectomy (BSO) prevents gynecological cancer in Lynch syndrome.8 The Manchester International Consensus Group strongly recommended that risk-reducing hysterectomy and BSO are offered no earlier than 35–40 years of age, following completion of childbearing in path_MLH1, path_MSH2, and path_MSH6 heterozygotes but the data supporting such recommendations are not strong, and various practices currently exist. There was insufficient evidence to strongly recommend risk-reducing surgery for path_PMS2 heterozygotes.6
In this report, we determine the impact on cancer incidence and mortality of RRS at different ages in heterozygotes of pathogenic MMR variants.
MATERIALS AND METHODS
The PLSD is an international, multicenter, prospective observational study without a control group. The PLSD design and its inclusion criteria have been described previously in detail.1,3,4,9,10
In brief, path_MMR heterozygotes, including probands and their relatives, were recruited for prospective follow-up in each participating center. Genetic variants were assumed to be inherited and were found by genetic testing either prior to, at, or after inclusion for follow-up. Inclusion was from the first prospectively planned and completed colonoscopy, and all recruits had subsequent follow-up of one year or more. Any cancers that were diagnosed before or at the same age as the first prospectively planned and completed colonoscopy were scored as previous cancers. Time to first cancer after inclusion was calculated for each organ or groups of organs. Only heterozygotes with pathogenic variants confirmed as class 4 or 5 (clinically actionable) in the International Society for Gastrointestinal Hereditary Tumors (InSiGHT) database (https://databases.lovd.nl/shared/genes) were included. Each patient was censored at the age at which the last information was available, which might have been a colonoscopy, any other clinical examination, a report from an examination done by others, or information that the patient had died, whichever came last. Observation time was censored at organ removal (therapeutic or prophylactic) when calculating incidences for cancer in specific organs.1
Impact on cancer incidence of risk-reducing hysterectomy and/or BSO by age and gene
The inclusion criteria for calculating the endometrial and ovarian cancer risks were (1) female, (2) heterozygotes with pathogenic (class 4 or 5) MMR variant as classified in the InSiGHT database (http://insight-database.org/), (3) no previous hysterectomy or BSO, and (4) aged 25 to 74 years at start of follow-up. The following information was used for analyses: age at last observation, incident endometrial and/or ovarian cancer, path_MMR variant, age at hysterectomy, and age at oophorectomy. In this report, we assume the oophorectomies undertaken were all BSO.
Endometrial and ovarian cancer risks are reported by 5-year age groups. These risks may be considered to represent cancers that would have been prevented if surgery had been undertaken before the ages concerned.
All risks used for calculations and their 95% confidence intervals are derived from our previous publications.1,2,3,4 Briefly, annual incidence rates (AIRs) by age were calculated in 5-year cohorts from 25 to 75 years of age. Cumulative incidence, denoted by Q, was computed starting at age 25, assuming zero incidence rate before age 25, using the formula Q(age) = Q(age − 1) + [1 − Q(age − 1)] × AIR(age) where AIR(age) is the annual incidence rate as estimated from the corresponding 5-year interval. The observed AIRs and cumulative incidence of endometrial and/or ovarian cancer in the current data set have not been described previously and are now presented here in the Supplementary file.
Risk of dying from endometrial or ovarian cancer
As in all previous PLSD reports, cancer incidence at 25 years of age (the minimum age from which PLSD collects prospective data) was assumed to be zero. In this report, we provide estimates of the risk of dying following endometrial or ovarian cancer, stratified by MMR gene from 25 to 69 years of age. As displayed at our interactive website (www.plsd.eu), the confidence intervals for these measures are wide for patients with heterozygous path_MSH6 and path_PMS2 variants, and the point estimates of risks for patients with these genotypes must be used with caution.
Survival after cancer was estimated by the Kaplan–Meier survival function as crude survival from age at diagnosis until last observation or death. All the AIRs and cumulative incidences are prospectively observed empirical observations, while the survival following endometrial and/or ovarian cancer was calculated as follows: at any given age for cumulative incidences in the tables for endometrial or ovarian cancer separately, we calculated the relative risk for having endometrial or ovarian cancer as the incidence of the one divided by the sum of the two incidences.
Survival after endometrial and/or ovarian cancer was calculated as follows. The following observed factors (with acronyms) were entered into the calculations: risk of endometrial cancer (ECrisk), risk of ovarian cancer (OCrisk), risk of ovarian and/or endometrial cancer (ECOCrisk), survival after endometrial cancer (ECsurvival), and survival after ovarian cancer (OCsurvival). The three former were age-dependent while the two latter were the same for all ages. From the two latter, the difference between the survival for ovarian and endometrial cancer (SURVdiff) was (ECsurvival – OCsurvival) = 5%, which was the same for all ages. For each age cohort given in the table, the fraction of endometrial cancer (ECfraction) was calculated as the risk for endometrial cancer divided by the sum of the risks for endometrial and ovarian cancer as ECrisk/(ECrisk + OCrisk). OC survival was lower than EC survival and the survival when ovarian and/or endometrial cancer was scored as an event; the interpolated combined survival indicated in the table was calculated as OCsurvival + SURVdiff *ECfraction for all age groups.
RESULTS
Survival after endometrial or ovarian cancer
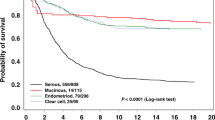
There were 58, 61, 18, and 4 cases of prospectively observed endometrial cancer included in the survival analyses in path_MLH1, path_MSH2, path_MSH6, and path_PMS2 heterozygotes, respectively. There were 22, 23, 1, and 1 prospectively detected ovarian cancer cases included in the survival analyses in path_MLH1, path_MSH2, path_MSH6, and path_PMS2 heterozygotes, respectively. The average for all cases was used to estimate survival for all heterozygotes in this report, but numbers of path_MSH6 and path_PMS2 heterozygotes were too low for us to determine whether the average survival pertains to these heterozygotes. The numbers of cases were also too few to permit calculations of survival by the age at which cancer occurred.
Estimates of five- and ten-year survival after endometrial or ovarian cancer in LS, but not stratified by gene, have been published previously.1 Figure 1 presents survival by gene. As illustrated, there were no significant differences between the genes. After a few early deaths, the curves for both endometrial and ovarian cancer survival flatten out. This is in contrast to the lower reported survival in path_BRCA1/2-associated or sporadic ovarian cancer cases for which the survival curve does not flatten out, although deaths beyond 5 years in BRCA1/2 cases are usually predicted by recurrence before that time.11
Impact on cancer incidence and mortality of risk-reducing hysterectomy and/or BSO by age and gene
Among the heterozygotes included in the last PLSD report1 there were 7838 observed female years for path_MLH1 heterozygotes, 5487 for path_MSH2, 1614 for path_MSH6, and 862 for path_PMS2 that met the selection criteria for the current study.
In Table 1 and Fig. 2, the risks for endometrial cancer from 25 up to 40, 50, 60, or 70 years of age are given by gene for patients who did not have surgery before each respective age cutoff. Risks from 40, 50, and 60 up to 70 years of age are given to indicate the potential for endometrial cancers to be prevented if hysterectomy is undertaken at these ages. The risks for developing cancer in each 10-year cohort are also given. In Table 2, the corresponding risks for ovarian cancer by age and gene are given. The combined risks for developing and dying from gynecological cancers by age and gene in the absence of risk-reducing hysterectomy and/or BSO are described in Table 3.
If risk-reducing hysterectomy were performed at 25 years of age, endometrial cancer before 50 years would be prevented in 15%, 18%, 13%, and 0%, in patients with heterozygous path_MLH1, path_MSH2, path_MSH6, and path_PMS2 variants, respectively, and death in 2%, 2%, 1%, and 0%. If risk-reducing BSO had been performed at 25 years of age, this would have prevented the observed risks of ovarian cancer to age 50 years of 6%, 11%, 2%, and 0% in patients with heterozygous path_MLH1, path_MSH2, path_MSH6, and path_PMS2 variants, respectively. Correspondingly, the observed ovarian cancer death risks by age 50 years of 1%, 2%, 0%, and 0% would have been prevented (Tables 1 and 2).
Risk-reducing hysterectomy at 40 years of age was estimated to prevent endometrial cancer by 50 years in 13%, 16%, 11%, and 0% of patients and death in 1%, 2%, 1%, and 0% for path_MLH1, path_MSH2, path_MSH6, and path_PMS2 heterozygotes, respectively. Similarly, BSO carried out at 40 years of age was estimated to prevent ovarian cancer before 50 years of age in 4%, 8%, 0%, and 0%, and to prevent death before 50 years in 1%, 1%, 0%, and 0%, respectively.
DISCUSSION
In this report, we describe the consequences of RRS by age and gene on incident gynecological cancer risk and associated deaths using observational data from the PLSD from 25 to 69 years of age for different intervention and observation endpoints. Our intention is to empower individual path_MMR heterozygotes to make an informed choice regarding whether or not to have risk-reducing gynecological surgery, and the optimal timing for this.
The results in Tables 1, 2, and 3 showing the consequences of having or not having hysterectomy and/or BSO at various ages demonstrate for path_MLH1, path_MSH2, and path_MSH6 heterozygotes a small cumulative cancer risk (2%) up to 40 years of age, and a more substantial risk (1.1% to 2.5% annual incidence)1 for endometrial cancer from 40 years of age onward. For these patients, the cumulative risk for ovarian cancer from 25 to 50 years is 6%, 11%, and 2% respectively, which combined with the average mortality, which is substantially lower than in BRCA1/2-associated or sporadic ovarian cancer, indicate a risk of dying from a premenopausal ovarian cancer to be 1%, 2%, and 0%, respectively. There is also a risk for postmenopausal ovarian cancer. Interpretation of estimates for RSS-associated endometrial and ovarian cancer survival benefit indicates that the absolute reduction in risk of cancer death achieved by very early RRS is small. Performing RRS on 25-year-olds instead of 40-year-olds yields incidence benefits of 0–3%, depending on the path_MMR gene, for endometrial and ovarian cancer mortality. These risk estimates are the best we currently have for informing the outcome of premenopausal BSO.
For path_PMS2 heterozygotes, there is no demonstrable risk for premenopausal endometrial or ovarian cancer, and therefore no argument for considering premenopausal RRS. Similarly, no increase in risk for postmenopausal ovarian cancer has been demonstrated in path_PMS2 heterozygotes and therefore there is no argument to consider postmenopausal BSO in this group differently from the general population.1,12
The cumulative risks for endometrial cancer in path_MLH1, path_MSH2, and path_MSH6 heterozygotes illustrated in Fig. 1 may give the impression that the annual incidence rates are substantially lower at older ages. As seen in Table 1, however, this is not so: the risk for endometrial cancer remains high at older ages. Figure 1 shows the typical S-shaped curves generated by conditional probabilities when risk initially increases with age. Because there are fewer older female heterozygotes who have not had endometrial cancer (or hysterectomy), residual risk at older ages results in a lower number of cancer cases than at younger ages, despite high annual incidence among older heterozygotes who have not already had cancer. The higher the risk in younger heterozygotes, the more pronounced this effect will be. Similarly, the combined cumulative incidence by age for endometrial or ovarian cancer as seen in Table 3 is slightly lower than the sum of the two as presented in Tables 1 and 2, because standard treatment of the one removes the risk of having the other at a later time.
While Tables 1 and 2 indicate risks for cancer and survival by age and gene at entry into each age group, any patient may input her actual age and specific genetic variant into the interactive website www.plsd.eu, which will return the risk for cancer in any organ from her current age to any future selected age. From this, one may calculate the risk of dying from that cancer using our previously published survival estimates for LS patients who are affected by that cancer. The figures derived are point estimates and should be interpreted with appropriate caution.
Daily intake of acetyl-salicylic acid (aspirin) has been demonstrated to reduce colon cancer risk in heterozygotes for path_MMR variants by about 50%.12 A recent study also demonstrates a reduction in endometrial cancer incidence in heterozygotes for path_MMR variants taking acetyl-salicylic acid.13 The results in both of these reports were not stratified by MMR gene or age. The reduced cancer risk was a long-term effect and did not achieve statistical significance for endometrial cancer alone.
This report calculates the impact of RRS on gynecological cancer risk in path_MMR heterozygotes according to age and affected MMR gene, and reports an estimate of a survival benefit in terms of deaths that are actually prevented by RRS. Our calculations are based on the largest international LS database in the world, reporting 15,800 prospective observation years for female path_MMR heterozygotes. The prospective registration of incident cancers and associated deaths minimizes ascertainment bias.
There are some limitations to the current study. Low number of patients with path_MSH6 and path_PMS2 variants may reflect that they are infrequently identified by the Amsterdam or Bethesda criteria and are infrequently subjected to genetic testing.14 With the advent of universal screening of colorectal and endometrial cancers for LS, this situation is likely to change.6 We restricted our analysis to report the prospectively observed endometrial and ovarian cancer incidence and survival in women who had not had prophylactic RRS to provide a robust analysis of cancer risk and associated deaths using observational data from the PLSD. We have not investigated for endometrial or ovarian cancer after RRS. When considering survival, it must be remembered that the results presented here were obtained prior to use of immunotherapy for microsatellite unstable tumors: future treatment modalities may further improve the survival, which is already much better than in sporadic or BRCA-associated ovarian cancer. Improved imaging and liquid biopsy may make early diagnosis and treatment more effective in future. We have assumed that all bilateral oophorectomies were BSO because type of RRS was not included in our data call.
There is a time-trend bias in the uptake of risk-reducing hysterectomy and BSO: older women may not have had the same option of early risk-reducing surgery that is advocated and available today (and they may not have known they were at risk when they were younger) and the uptake among older women may not be representative of what younger heterozygotes choose today. Because of the inherent time-trend bias, from which no statistical procedures can escape, we considered it inappropriate to investigate the reported uptake of these interventions using more sophisticated statistical methods.
The offer of RRS is currently recommended for women with path_MMR variants no earlier than 35–40 years of age6 (also see Seppala et al.,7 patient 2286). Our intention is to empower individual path_MMR heterozygotes to make an informed choice. We do not make management recommendations; rather, we promote personal choice for each path_MMR heterozygote based on current data. Since the figures derived are point estimates and should be interpreted with appropriate caution, the use of this information in decision making should be discussed with appropriately trained health-care professionals.
Conclusions
Our findings may be useful when disclosing results of genetic testing for path_MMR variants, since female heterozygotes have to decide which health-care options to select to manage their gynecological cancer risks. Clinical guideline recommendations should now be updated to take account of empirically observed risks for endometrial or ovarian cancers in path_MMR heterozygotes by age and gene.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. We have published a website (www.lscarisk.org) on which cancer risks for all published data can be reviewed and calculated in graphic form.
References
Dominguez-Valentin, M. et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet. Med. 22, 15–25 (2019).
Moller, P. et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut 66, 1657–1664 (2017).
Moller, P. et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 66, 464–472 (2017).
Moller, P. et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 67, 1306–1316 (2018).
Ryan, N. A. J. et al. Association of mismatch repair mutation with age at cancer onset in lynch syndrome: implications for stratified surveillance strategies. JAMA Oncol. 3, 1702–1706 (2017).
Crosbie, E. J. et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet. Med. 21, 2390–2400 (2019).
Seppala T. T. et al. European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br. J. Surg. https://doi.org/10.1002/bjs.11902 (2020).
Schmeler, K. M. et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N. Engl. J. Med. 354, 261–269 (2006).
Seppala, T. et al. Colorectal cancer incidence in path_MLH1 carriers subjected to different follow-up protocols: a prospective lynch syndrome database report. Hered. Cancer Clin. Pract. 15, 18 (2017).
Seppala, T. T. et al. Lack of association between screening interval and cancer stage in Lynch syndrome may be accounted for by over-diagnosis; a prospective Lynch syndrome database report. Hered. Cancer Clin. Pract. 17, 8 (2019).
Finch, A. et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 296, 185–192 (2006).
Burn, J. et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 378, 2081–2087 (2011).
Burn, J. et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet 395, 1855–1863 (2020).
Moller, P. The prospective lynch syndrome database reports enable evidence-based personal precision health care. Hered. Cancer Clin. Pract. 18, 6 (2020).
Dominguez-Valentin, M. et al. Survival by colon cancer stage and screening interval in Lynch syndrome: a prospective Lynch syndrome database report. Hered. Cancer Clin. Pract. 17, 28 (2019).
Acknowledgements
The study was supported by a Pink Ribbon grant (194751) from Den Norske Kreftforening to E.H. We express our gratitude to Heikki Järvinen, Beatrice Alcala-Repo, Teresa Ocaña, María Pellisé, Sabela Carballal, Liseth Rivero, Lorena Moreno, Gerhard Jung, Antoni Castells, Joaquin Cubiella, Laura Rivas, Luis Bujanda, Inés Gil, Jesús Bañales, Catalina Garau, Rodrigo Jover, María Dolores Picó, Xavier Bessa, Cristina Álvarez, Montserrat Andreu, Carmen Poves, Pedro Pérez Segura, Lucía Cid, Marta Carrillo, Enrique Quintero, Ángeles Pizarro, Marta Garzón, Adolfo Suárez, Inmaculada Salces, Daniel Rodriguez-Alcalde, Judith Balmaña, Adrià López, Nuria Dueñas, Gemma Llort, Carmen Yagüe, Teresa Ramón i Cajal, David Fisas Masferrer, Alexandra Gisbert Beamud, Consol López San Martín, Maite Herráiz, Pilar Pérez, Cristina Carretero, Maite Betés, Marta Ponce, Elena Aguirre, Nora Alfaro, Carlos Sarroca, and Marianne Haeusler for their efforts over many years. We also thank the Finnish Cancer Foundation, Jane and Aatos Erkko Foundation, Emil Aaltonen Foundation, Finnish Medical Foundation, Instrumentarium Science Foundation, Sigrid Juselius Foundation, and the Norwegian Cancer Society (contract 194751–2017) for funding. D.G.E. and E.J.C. are supported by the all Manchester National Institute for Health Research (NIHR) Biomedical Research Center (IS-BRC-1215–20007) and J.R.S. by Health and Care Research Wales through Wales Gene Park. N.R. was a Medical Research Council Doctoral Research Fellow (MR/M018431/1). Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number UM1CA167551 and through cooperative agreements with the following The Colon Cancer Family Registry (CCFR) centers: Australasian Colorectal Cancer Family Registry (NCI/NIH U01 CA074778 and U01/U24 CA097735), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (NCI/NIH U01/U24 CA074800), Ontario Familial Colorectal Cancer Registry (NCI/NIH U01/U24 CA074783), Seattle Colorectal Cancer Family Registry (NCI/NIH U01/U24 CA074794), University of Hawaii Colorectal Cancer Family Registry (NCI/NIH U01/U24 CA074806 and R01 CA104132 to L.L.), USC Consortium Colorectal Cancer Family Registry (NCI/NIH U01/U24 CA074799). The German Consortium for Familial Intestinal Cancer has been supported by grants from the German Cancer Aid. Data collection from Wales, UK was supported by the Wales Gene Park. This work was cofunded by the European Regional Development Fund (ERDF). M.N. and S.W.t.B were supported by a grant from the Dutch Cancer Society (DCS), grant number UL 2017-8223. G.C., M.P., and J.B.V. were funded by the Spanish Ministry of Economy and Competitiveness and cofunded by FEDER funds (A Way to Build Europe) (grant SAF2015–68016-R), CIBERONC, the Government of Catalonia (grant 2017SGR1282).
Author information
Authors and Affiliations
Contributions
MD-V, EC and PM designed the study and wrote the manuscript with TTS and JRS. PM calculated the results. All others: acquisition of data, commenting and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Reinhard Büttner: Co-founder and the chief scientific officer of Targos Mol Path Inc., Kassel, Germany. Sir Joh Burn: Has a patent for high speed low cost tumour profiling pending to John Burn and QuantuMDx.
Ethics declaration
All reporting centers exported de-identified data to the PLSD and the patients had been followed up prospectively according to local clinical guidelines, as previously described.1,2,3,4,9,10,15
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dominguez-Valentin, M., Crosbie, E.J., Engel, C. et al. Risk-reducing hysterectomy and bilateral salpingo-oophorectomy in female heterozygotes of pathogenic mismatch repair variants: a Prospective Lynch Syndrome Database report. Genet Med 23, 705–712 (2021). https://doi.org/10.1038/s41436-020-01029-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-01029-1
This article is cited by
-
UK recommendations for the management of transgender and gender-diverse patients with inherited cancer risks
BJC Reports (2023)
-
Risk-reducing surgery for individuals with cancer-predisposing germline pathogenic variants and no personal cancer history: a review of current UK guidelines
British Journal of Cancer (2023)
-
Dominantly inherited micro-satellite instable cancer – the four Lynch syndromes - an EHTG, PLSD position statement
Hereditary Cancer in Clinical Practice (2023)
-
The Prospective Lynch Syndrome Database: background, design, main results and complete MySQL code
Hereditary Cancer in Clinical Practice (2022)