Abstract
Purpose
To assess the utility of globotriaosylsphingosine (lyso-Gb3) for clinical monitoring of treatment response in patients with Fabry disease receiving migalastat.
Methods
A post hoc analysis evaluated data from 97 treatment-naive and enzyme replacement therapy (ERT)–experienced patients with migalastat-amenable GLA variants from FACETS (NCT00925301) and ATTRACT (NCT01218659) and subsequent open-label extension studies. The relationship between plasma lyso-Gb3 and measures of Fabry disease progression (left ventricular mass index [LVMi], estimated glomerular filtration rate [eGFR], and pain) and the relationship between lyso-Gb3 and incidence of Fabry-associated clinical events (FACEs) were assessed in both groups. The relationship between changes in lyso-Gb3 and kidney interstitial capillary (KIC) globotriaosylceramide (Gb3) inclusions was assessed in treatment-naive patients.
Results
No significant correlations were identified between changes in lyso-Gb3 and changes in LVMi, eGFR, or pain. Neither baseline lyso-Gb3 levels nor the rate of change in lyso-Gb3 levels during treatment predicted FACE occurrences in all patients or those receiving migalastat for ≥24 months. Changes in lyso-Gb3 correlated with changes in KIC Gb3 inclusions in treatment-naive patients.
Conclusions
Although used as a pharmacodynamic biomarker in research and clinical studies, plasma lyso-Gb3 may not be a suitable biomarker for monitoring treatment response in migalastat-treated patients.
Similar content being viewed by others
INTRODUCTION
Fabry disease (OMIM 301500) is a rare, progressive X-linked lysosomal disorder caused by pathogenic variants in the α-galactosidase A gene (GLA), resulting in functional deficiency of α-galactosidase A (α-Gal A) and accumulation of glycosphingolipids within lysosomes, including globotriaosylceramide (Gb3) and globotriaosylsphingosine (lyso-Gb3).1,2 Glycosphingolipids accrue in many cell types, such as capillary endothelial, renal, cardiac, and nerve cells1,2 and several studies implicate activation of Toll-like receptors as a trigger of inflammatory and fibrotic cascades,3 which ultimately lead to multisystem dysfunction and death from cardiac disease, renal failure, or cerebrovascular disease.4 Lyso-Gb3 is the hydrophilic deacylated form of Gb3 and is detected at high levels in plasma in patients with classic Fabry disease.2,5 Various analogs of lyso-Gb3 with modifications to the sphingosine chain were also detected in plasma of patients with Fabry disease.6 It has been reported in mouse models that lyso-Gb3 accumulates most in the liver and spleen, organs that are not affected in Fabry disease.2 Although elevated intracellular Gb3 and lyso-Gb3 are considered to trigger inflammation,3 the persistence of altered cellular signaling subsequent to Gb3 clearance indicates that inflammation, when activated, could be uncoupled from substrate accumulation,7 and that at some point, the pathologic consequences are irreversible.
Approved treatments for Fabry disease include intravenous enzyme replacement therapy (ERT) and the oral pharmacological chaperone migalastat.8,9,10 ERT compensates for α-Gal A deficiency in patients with Fabry disease and is delivered through intravenous infusion.8,9 Migalastat is an orally administered small molecule that binds to and stabilizes endogenous α-Gal A in patients with migalastat-amenable GLA variants, facilitating lysosomal trafficking and restoration of native enzyme activity.10,11 As a small molecule, migalastat has broad tissue distribution and penetration.10,12 In phase 3 clinical studies, migalastat treatment effectively decreased disease substrates, stabilized renal function, reduced left ventricular mass index (LVMi), and improved gastrointestinal symptoms in patients with Fabry disease and amenable variants.13,14
Given that treatment options with different mechanisms of action exist and our understanding of Fabry pathophysiology and genetics is evolving, there is an increasing need for prognostic biomarkers to monitor and evaluate therapeutic efficacy and disease progression. A prognostic biomarker is one that is validated to identify the likelihood of a clinical event or progression of disease.15 Although some biomarkers have been identified that show diagnostic and pharmacodynamic value, no prognostic biomarkers have been validated for any Fabry disease therapy.15 Lyso-Gb3 is frequently and appropriately used for primary screening and diagnosing patients with Fabry disease. Studies have demonstrated that lyso-Gb3 effectively identifies unrecognized Fabry disease probands in patients referred from multispecialty clinics16 and detects clinically relevant Fabry disease phenotypes (classic vs. late onset).17 In cross-sectional studies, plasma lyso-Gb3 levels were found to associate with disease severity in patients with Fabry disease.18,19 Relationships between lyso-Gb3 and clinical manifestations of Fabry disease were also examined based on cross-sectional data.19,20,21 For example, plasma lyso-Gb3 adjusted for sex and age correlated with LVMi in a cross-sectional study of untreated patients with Fabry disease and the late-onset variant IVS4+919G>A.19 Similarly, plasma lyso-Gb3 correlated with left ventricular hypertrophy and myocardial fibrosis in patients with Fabry disease in recent prospective multicenter studies.20,21 However, these studies did not address how changes in lyso-Gb3 may relate to treatment outcomes (e.g., LVMi and estimated glomerular filtration rate [eGFR]) over time.
Although lyso-Gb3 has commonly been used in treatment monitoring, it has not been validated for this purpose, and few longitudinal studies have evaluated the association of lyso-Gb3 with treatment outcomes.22,23 One study showed that neither the lyso-Gb3 concentration at baseline, lyso-Gb3 concentration during treatment, absolute decrease of lyso-Gb3, nor the relative decrease of lyso-Gb3 predicted the risk of clinical events in patients receiving ERT.22 In addition, the absence or presence of end-organ damage was not predicted by absolute lyso-Gb3 levels, and undetectable or low lyso-Gb3 levels in patients with the late-onset presentation of Fabry disease did not protect patients from end-organ clinical events.24 It is increasingly recognized that the mechanism of Fabry disease is more complex than previously thought and that substrate accumulation alone does not explain disease severity and therapeutic response to a given treatment.3 However, the downstream effects of substrate accumulation are not well understood.
Given the desire for validated biomarkers of clinical disease progression in treated patients with Fabry disease, the value of lyso-Gb3 as a prognostic biomarker needs to be evaluated in migalastat-treated patients. Here, we examined plasma lyso-Gb3 profiles in treatment-naive and ERT-experienced patients with migalastat-amenable GLA variants in the phase 3 clinical studies FACETS (NCT00925301)13 and ATTRACT (NCT01218659)14 and subsequent long-term open-label extension studies to assess the relationship between plasma lyso-Gb3 and measures of clinical disease progression of Fabry disease (LVMi, eGFR, and pain), and Fabry-associated clinical events (FACEs) over time. We also aimed to confirm the utility of lyso-Gb3 as a pharmacodynamic biomarker by assessing its relationship with kidney interstitial capillary (KIC) Gb3, a commonly used, “reasonably likely surrogate endpoint” for accelerated approval of treatments for Fabry disease in the United States.25
MATERIALS AND METHODS
Ethics statement
FACETS, ATTRACT, AT1001-041, and AT1001-042 were all designed and monitored in accordance with the ethical principles of Good Clinical Practice guidelines and the Declaration of Helsinki.13,14 The clinical study protocols were reviewed and approved by the appropriate Independent Ethics Committee/Institutional Review Board at each study site. All participants provided written informed consent prior to initiation of any studies.
Study design and patients
This post hoc analysis includes data from treatment-naive and ERT-experienced adult patients with Fabry disease who enrolled in the phase 3 clinical studies FACETS13 (NCT00925301) and ATTRACT14 (NCT01218659), respectively. Briefly, FACETS comprised a 6-month randomized, double-blind, placebo-controlled phase, followed by a 6-month open-label extension (OLE) phase with crossover of patients in the placebo arm to receive migalastat 150 mg every other day (QOD), and a 12-month migalastat treatment extension phase. ATTRACT was an open-label, randomized study comprising an 18-month active-controlled (ERT), randomized phase and a 12-month optional OLE phase with crossover of patients in the ERT arm to receive migalastat 150 mg QOD. Data were collected during the phase 3 trials and the long-term OLE safety and efficacy studies AT1001-041 (NCT01458119) and AT1001-042 (NCT02194985) (Fig. S1). Eligibility criteria and study designs for FACETS and ATTRACT were published previously.13,14 This analysis included all patients who had an amenable GLA variant based on the Good Laboratory Practice–validated migalastat amenability assay in human embryonic kidney cells and had received at least one dose of migalastat.
Assessments
Plasma lyso-Gb3 and measures of Fabry disease progression including LVMi, eGFR, and pain were assessed in both ERT-naive and ERT-experienced patients. Plasma lyso-Gb3 levels were analyzed on a research basis at Amicus Therapeutics, Inc. by liquid chromatography–tandem mass spectrometry using plasma samples collected at study enrollment and every 6 months thereafter; LVMi was calculated based on echocardiography measures assessed through blinded, centralized evaluation;13,14 eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration equation (eGFRCKD-EPI); and worst pain in 24 hours was collected using the Brief Pain Inventory Short Form in patients with Fabry disease in the FACETS and ATTRACT studies,13,14 analyzing only responses to the question on the worst pain over a 24-hour period.
The relationship between lyso-Gb3 and FACEs was also analyzed. FACEs occurring during the trial were defined previously in the ATTRACT study and included cardiac, renal, and cerebrovascular events, and death.14 Cardiac, renal, and cerebrovascular events were defined as follows:
-
Cardiac events
-
Myocardial infarction
-
Unstable cardiac angina, as defined by the American College of Cardiology/American Heart Association national practice guidelines
-
New symptomatic arrhythmia requiring antiarrhythmic medication, direct current cardioversion, pacemaker, or defibrillator implantation, or
-
Congestive heart failure, New York Heart Association class III or IV
-
-
Renal events
-
A decrease in eGFRCKD-EPI ≥ 15 mL/min/1.73 m2, with the decreased eGFR <90 mL/min/1.73 m2 relative to baseline, or
-
An increase in 24-hour urine protein ≥33%, with elevated protein ≥300 mg relative to baseline
-
-
Cerebrovascular events
-
Stroke, or
-
Transient ischemic attack
-
KIC Gb3 inclusions were assessed quantitatively using the Barisoni Lipid Inclusion Scoring System in biopsy samples from ERT-naive patients as described previously.13,26
Statistical analyses
For this post hoc analysis, baseline (month 0) was defined as the beginning of migalastat treatment. The data cutoff date for the ongoing AT1001-042 study was 25 May 2019.
Relationships between changes in lyso-Gb3 and changes in measures of disease progression (i.e., LVMi, eGFR, and pain) were assessed for all patients with amenable variants and migalastat exposure. In addition, the relationship between baseline values of lyso-Gb3 and LVMi, eGFR, and pain was evaluated. Three subgroup analyses were performed in which data were stratified by prior ERT treatment status (naive or ERT-experienced), sex, or age (≤40 and >40 years). Spearman rank correlation coefficients and P values were calculated to assess correlations between changes in lyso-Gb3 and changes in LVMi, eGFR, or pain at months 12, 18, 24, 30, and 36 for ERT-naive and ERT-experienced patients during migalastat treatment, specifically. Correlations between changes in lyso-Gb3 and changes in KIC Gb3 were assessed at months 6 and 12 only in ERT-naive patients during migalastat treatment.
Relationships between variables over time were assessed in longitudinal analyses via random coefficient mixed models. A separate set of regression coefficients was fitted for each response variable (LVMi, eGFR, and pain) and the correlations among these random coefficients were examined in a pairwise manner with regression coefficients for lyso-Gb3. The model was implemented via PROC MIXED using SAS Enterprise Guide version 8.1 (SAS Institute; Cary, NC, USA). The method assesses if the slopes for the two variables tested (e.g., lyso-Gb3 and LVMi) are independent.27
Cox proportional hazard models were used to assess any relationships between lyso-Gb3 and the incidence of FACEs. This analysis was performed for all patients and patients who had continued migalastat therapy for ≥24 months. For patients with recurrent events, only the first events were analyzed. The data were right censored if a patient dropped out of the study before an event occurred (at the time of dropout) or had experienced no event at the end of the follow-up (May 25, 2019). Modeling was conducted using SAS Enterprise Guide version 8.1, and the following covariates were included in this analysis: age, time from diagnosis, presence of FACEs prior to the start of migalastat therapy, urine protein values at baseline, LVMi at baseline, and eGFR at baseline.
Previous cardiac and cerebrovascular events were identified based on Medical Dictionary for Regulatory Activities codes for the preferred terms from medical history with the exception of arrhythmias, which were evaluated by a physician. Previous renal events were defined as baseline eGFRCKD-EPI < 60 mL/min/1.73 m2 or baseline urine protein ≥300 mg, or any renal events as identified by a physician in the medical history. All variables were introduced into the Cox model tested, P values were calculated using Wald chi-square statistics, and P < 0.05 was considered significant.
The statistical analyses were not adjusted for multiplicity.
RESULTS
Patient demographics and baseline characteristics
Patient demographics and disease characteristics at baseline (start of migalastat) are shown for all 97 patients included in the analysis, and by prior treatment status and sex in Table 1. Overall, mean (standard deviation) age was 46.2 (13.1) years, 60 (61.9%) patients were female, and 86 (88.7%) patients had ≥1 prior FACE. At baseline, LVMi, eGFR, and history of previous clinical events were generally comparable between patient subgroups. The upper limit of the normal reference range for plasma lyso-Gb3 was 1.19 nmol/L.14 The median (range) duration of migalastat exposure was 5.1 (0.1–8.5) years in the overall patient group, and the median (range) duration of migalastat exposure was 6.5 (0.1–8.5) years in treatment-naive patients and 5.0 (0.1–7.2) years in ERT-experienced patients (Table 1).
Relationship between plasma lyso-Gb3 and measures of Fabry disease progression
At baseline, plasma lyso-Gb3 levels were not correlated with eGFR or worst pain in 24 hours in treatment-naive and ERT-experienced patients (Table S1). When analyzed by sex or age, no correlations were identified between lyso-Gb3 and eGFR; however, a significant correlation between baseline lyso-Gb3 and baseline pain was identified in male patients (Spearman correlation unadjusted P = 0.0038). Baseline lyso-Gb3 was shown to correlate with baseline LVMi in both ERT-naive and ERT-experienced patients (unadjusted P = 0.0002 and unadjusted P = 0.0016, respectively) and patients aged ≤40 and >40 years (unadjusted P = 0.0007 and unadjusted P = 0.0003, respectively). In contrast, no correlation was identified between baseline lyso-Gb3 and baseline LVMi when patients were analyzed by sex.
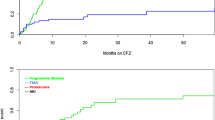
During migalastat treatment, no correlation was identified between changes from baseline in lyso-Gb3 and changes from baseline in LVMi, eGFR, or worst pain in 24 hours at any timepoint analyzed in treatment-naive and ERT-experienced patients (Table 2). When analyzed by sex, no correlations between changes in lyso-Gb3 and changes in LVMi, eGFR, or worst pain in 24 hours were identified in male or female patients for any timepoint with one exception. A correlation was identified between lyso-Gb3 and eGFR at month 18 in male patients (unadjusted P = 0.03). Similarly, when analyzed by age, no correlations were identified between lyso-Gb3 and LVMi, eGFR, and pain at any timepoint assessed except for a correlation between lyso-Gb3 and eGFR at month 12 in patients aged >40 years (unadjusted P = 0.02). The individual changes from baseline in lyso-Gb3 were plotted against changes from baseline in LVMi, eGFR, or worst pain in 24 hours at selected timepoints by treatment status (Fig. 1), and no trends between lyso-Gb3 and LVMi, eGFR, or pain were observed.
(a) Relationship between changes in lyso-Gb3 versus changes in LVMi. (b) Relationship between changes in lyso-Gb3 vs. changes in eGFR. (c) Relationship between changes in lyso-Gb3 versus changes in worst pain in 24 hours. eGFR estimated glomerular filtration rate, ERT enzyme replacement therapy, LVMi left ventricular mass index, lyso-Gb3 globotriaosylsphingosine, OLE open-label extension.
When assessing the rate of change in lyso-Gb3 and LVMi or eGFR during follow-up, no longitudinal correlation was identified in the overall patient group or subgroups stratified by prior ERT treatment status and sex (Table 3). A longitudinal correlation was identified between lyso-Gb3 and worst pain in 24 hours in the overall patient group (r = 0.82; unadjusted P < 0.01), ERT-experienced patients (r = 0.69; unadjusted P = 0.04), and male patients (r = 0.99; unadjusted P = 0.02), but not in treatment-naive or female patients (Table 3). When analyzed by age, no longitudinal correlation was identified between lyso-Gb3 and LVMi, eGFR, or pain in patients aged ≤40 years. However, a longitudinal correlation was identified between lyso-Gb3 and LVMi in patients aged >40 years (r = 0.63; unadjusted P = 0.02).
Relationship between plasma lyso-Gb3 and incidence of FACEs
Overall, FACEs occurred in 47 (48.5%) patients receiving migalastat treatment. When analyzed by ERT treatment status, FACEs occurred in 22 (45.8%) treatment-naive patients and 25 (51.0%) ERT-experienced patients while on migalastat treatment. Among male and female patients, 22 (59.5%) and 25 (41.7%) patients experienced FACEs, respectively.
When we assessed the ability of various baseline variables to predict the incidence of FACEs, only baseline eGFR was associated with the occurrence of FACEs during migalastat treatment (hazard ratio [HR]: 0.74 for every 10 mL/min/1.73 m2; unadjusted P = 0.02) (Table 4). Similarly, higher baseline eGFR was associated with a decreased incidence of FACEs when analyses were controlled for prior ERT treatment status (HR: 0.72 for every 10 mL/min/1.73 m2; unadjusted P = 0.02) or sex (HR: 0.74 for every 10 mL/min/1.73 m2; unadjusted P = 0.02). Neither lyso-Gb3 levels at baseline nor the rate of change in lyso-Gb3 levels during treatment predicted the occurrence of FACEs in the overall patient group (HR: 1.05 for every 10 nmol/L in baseline lyso-Gb3 levels; unadjusted P = 0.60 and HR: 1.02 for every 0.05 nmol/L/month in the rate of change in lyso-Gb3 level; unadjusted P = 0.62). Similarly, neither variable predicted FACEs when analyses were controlled for prior ERT treatment status (HR: 1.06; unadjusted P = 0.54 and HR: 1.00; unadjusted P = 0.96, respectively) or sex (HR: 1.05; unadjusted P = 0.56 and HR: 1.02; unadjusted P = 0.54, respectively).
Similar results were observed when the analysis was restricted to all patients who had received ≥24 months of migalastat treatment (Table 4). Higher baseline eGFR was associated with decreased incidence of FACEs (HR: 0.72 for every 10 mL/min/1.73 m2; unadjusted P = 0.02). Neither lyso-Gb3 baseline levels nor the rate of change in lyso-Gb3 levels was associated with the occurrence of FACEs (HR: 1.07; unadjusted P = 0.47 and HR: 1.02; unadjusted P = 0.54, respectively). In addition, neither was associated with the occurrence of FACEs when analyses were controlled for prior ERT treatment status (HR: 1.08; unadjusted P = 0.43 and HR: 1.01; unadjusted P = 0.78) or sex (HR: 1.08; unadjusted P = 0.40 and HR: 1.02; unadjusted P = 0.47) in this long-term treatment subgroup.
Relationship between plasma lyso-Gb3 and KIC Gb3
KIC Gb3 was only assessed in the FACETS study up to 12 months after which biopsies were not obtained. The relationship between plasma lyso-Gb3 and KIC Gb3 was evaluated in ERT-naive patients only. A correlation was identified between lyso-Gb3 and KIC Gb3 in ERT-naive patients at months 6 and 12 (unadjusted P < 0.01 and unadjusted P = 0.05, respectively) (Table 2). When patients were analyzed by sex, a correlation was identified in male patients at month 6 (unadjusted P = 0.04). However, no correlation was identified in female patients at either timepoint (unadjusted P = 0.07 and unadjusted P = 0.72, respectively). When patients were analyzed by age, a correlation was identified between lyso-Gb3 and KIC Gb3 in patients aged ≤40 and >40 years at month 6 only (unadjusted P = 0.002 and unadjusted P = 0.02, respectively).
DISCUSSION
There is skepticism about the usefulness of monitoring plasma lyso-Gb3 to evaluate treatment response in Fabry disease.22,24 Despite its utility for diagnosis, disease severity assessment, and as a pharmacodynamic biomarker,16,17,28 lyso-Gb3 values showed little association with treatment outcomes in ERT-treated patients (agalsidase alfa or agalsidase beta).22 In the current study, changes in plasma lyso-Gb3 levels did not correlate with changes in measures of clinical disease progression (LVMi, eGFR, or pain) in treatment-naive or ERT-experienced patients during migalastat treatment. However, without adjusting for multiplicity, lyso-Gb3 correlated with LVMi at baseline, and a longitudinal correlation was identified between lyso-Gb3 and LVMi in patients aged >40 years. Plasma lyso-Gb3 measurements, including levels at baseline or rate of change in lyso-Gb3 levels during treatment, did not predict FACEs in treatment-naive or ERT-experienced patients, suggesting that changes in plasma lyso-Gb3 may not predict therapeutic outcome and may have limited utility in clinical monitoring and decision making for migalastat-treated patients from this cohort.
These observations are in line with the evolving understanding of Fabry pathophysiology, which likely includes multiple disease mechanisms beyond substrate storage. In support of this, glycosphingolipid substrate clearance by ERT did not reverse several Fabry disease–associated pathophysiological processes in a recent study in cultured podocytes.7 Furthermore, the origin of plasma lyso-Gb3 is not well understood. Nevertheless, a possible biochemical relationship between Gb3 and lyso-Gb3 was revealed in a urine metabolomic study where the mass spectrometry fragmentation approach showed that methylated Gb3-related analogs might be intermediate compounds leading to Gb3 deacylation and lyso-Gb3 generation.29 Furthermore, studies in mouse models suggest that lyso-Gb3 is either actively formed or preferentially stored in the liver and spleen, organs not affected by Fabry disease.2,30,31 Elevated plasma lyso-Gb3 could be a “spillover” from these organs and therefore may not reflect the substrate levels in clinically relevant organs such as the heart, kidney, or peripheral nerves.1 In addition, as migalastat and lyso-Gb3 primarily occupy distinct compartments (lysosomes versus plasma),2,10 lyso-Gb3 may not be subject to catalysis by migalastat-stabilized α-Gal A.
Given that lyso-Gb3 generally did not correlate with measures of Fabry disease progression or predict the incidence of FACEs during migalastat treatment, our results confirm for migalastat what was already demonstrated for ERT regarding the poor value of lyso-Gb3 for monitoring treatment.22
Although these data suggest that lyso-Gb3 is not a suitable prognostic biomarker for Fabry disease, lyso-Gb3 and Gb3 are robust pharmacodynamic biomarkers commonly used in research of new treatments for Fabry disease.13,14,32 ERT and migalastat have effectively reduced plasma lyso-Gb3 levels in patients with Fabry disease.5,14,28 It was also observed in a retrospective study that among ERT-treated male patients with Fabry disease, those who developed antibodies against ERT had significantly higher plasma lyso-Gb3 levels than those without ERT antibodies (P = 0.02).33 In addition, ERT substantially reduced Gb3 inclusions in KIC and glomerular cells after 6 months of treatment in patients with Fabry disease,8,9 and migalastat decreased podocyte volume and partially cleared Gb3 inclusions in podocytes after 6 months of treatment in male patients with Fabry disease.34 These observations demonstrate a clear biological response in individuals treated with migalastat and ERT, which is the purpose of a pharmacodynamic biomarker.35 Consequently, KIC Gb3 has served as a “reasonably likely surrogate endpoint” and is the basis of regulatory approval for ERT and migalastat in the United States and continues to be used in clinical development programs.13,25,32 In this analysis, changes in lyso-Gb3 correlated with changes in KIC Gb3 in ERT-naive patients at months 6 and 12, supporting its utility as a pharmacodynamic biomarker in the clinical development of treatments for Fabry disease.
Several studies have suggested associations between lyso-Gb3 and manifestations of Fabry disease, including LVMi and myocardial fibrosis.19,20,21,23 However, it should be noted that these associations were found using cross-sectional data in either untreated patients19,21 or in heterogeneous patient populations in which a subset of patients received ERT.20,23 Indeed, our finding that baseline lyso-Gb3 levels correlated with baseline LVMi is consistent with previous report,19 but to our knowledge, no publication had explored the relationship between changes in lyso-Gb3 and LVMi during treatment. One study evaluated longitudinal changes in myocardial fibrosis in untreated patients and found baseline lyso-Gb3 was not a predictor of fibrosis during follow-up.21 Another study identified a trend toward a correlation between lyso-Gb3 and decline in pulmonary function with age as assessed by spirometry in a mixed population of ERT-treated and untreated patients with Fabry disease.23 Therefore, the value of lyso-Gb3 for treatment monitoring remains uncertain as these studies did not address the association between changes in lyso-Gb3 levels and treatment outcomes. However, the current analysis using longitudinal data and FACEs fills an important gap in research by evaluating the clinical utility of lyso-Gb3 for treatment monitoring of patients with Fabry disease receiving migalastat.
Study limitations include the fact that this is a post hoc analysis of data from trials not specifically designed to explore the research question and lack of adjustment for multiplicity in statistical analyses, which could be a source of bias considering the relatively small patient number and may account for significant correlations that were identified between lyso-Gb3 and KIC Gb3 and measures of Fabry disease progression in a subset of patients at certain timepoints. In addition, few patients had LVMi and pain data beyond 36 months of migalastat treatment, and longitudinal correlations between slopes of lyso-Gb3 and pain could not be calculated for female patients due to the small sample size. Pain measurements included the worst pain in 24 hours only, suggesting that these analyses may not have fully explored the relationship between lyso-Gb3 and general pain levels.
Although this is a post hoc analysis, patients were stratified by baseline lyso-Gb3 level to calculate Spearman correlation coefficients or longitudinal correlations. Furthermore, adjustment for baseline lyso-Gb3 and change over time was included in Cox proportional hazard models to assess FACEs. However, a prospective study that includes formal biomarker validation methodology would be informative.
Few studies have investigated potential prognostic biomarkers for Fabry disease progression and clinical response to treatment for the guidance of treatment decisions.22,23 Therefore, new biomarkers should be explored for monitoring treatment effect in Fabry disease. More studies are needed to confirm the findings of this study and explore the value of existing biomarkers including proteinuria/albuminuria,36 podocyturia,36 inflammatory markers such as tumor necrosis factor,20 potential biomarkers of renal disease including podocalyxin36 and fibroblast growth factor 23,37,38 and markers of cardiac disease.21,39 Moreover, future studies are warranted to assess any relationships between lyso-Gb3 analogs and Fabry disease severity given that lyso-Gb3 analogs constitute a substantial proportion of total lyso-Gb3 in plasma and urine of patients with Fabry disease.19,28 Proteomic and metabolomic profiling of plasma and/or urine samples of patients with Fabry disease compared with healthy controls may also identify potential biomarkers of Fabry disease progression.38
In conclusion, these post hoc analyses show that plasma lyso-Gb3 levels generally do not correlate with measures of disease progression (LVMi, eGFR, and pain) or predict FACEs in migalastat-treated patients regardless of their previous treatment history or sex. Our results confirm that lyso-Gb3 is not a prognostic biomarker of migalastat treatment response in patients with Fabry disease, a finding similar to what has been published for ERT.22 For patients receiving migalastat, the ongoing effectiveness of treatment should be determined based on the totality of biochemical and clinical evidence as well as patient-reported outcomes for consistency with current treatment monitoring guidelines.40 Clinical decision making must consider effectiveness, safety and tolerability, and patient preference.
Change history
08 November 2020
This Article was originally published under Nature Researchʼs License to Publish, but has now been made available under a [CC BY-NC-ND 4.0] license. The PDF and HTML versions of the Article have been modified accordingly.
20 November 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30.
Aerts JM, Groener JE, Kuiper S, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci USA. 2008;105:2812–2817.
Rozenfeld P, Feriozzi S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab. 2017;122:19–27.
Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med. 2009;11:790–796.
van Breemen MJ, Rombach SM, Dekker N, et al. Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim Biophys Acta. 2011;1812:70–76.
Dupont FO, Gagnon R, Boutin M, Auray-Blais C. A metabolomic study reveals novel plasma lyso-Gb3 analogs as Fabry disease biomarkers. Curr Med Chem. 2013;20:280–288.
Braun F, Blomberg L, Brodesser S, et al. Enzyme replacement therapy clears Gb3 deposits from a podocyte cell culture model of Fabry disease but fails to restore altered cellular signaling. Cell Physiol Biochem. 2019;52:1139–1150.
Fabrazyme [prescribing information]. Cambridge, MA: Sanofi Genzyme; 2018.
Replagal [summary of product characteristics]. Dublin, Ireland: Shire Pharmaceuticals Ltd; 2018.
Galafold [prescribing information]. Cranbury, NJ: Amicus Therapeutics Inc.; 2020.
Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005;19:12–18.
Khanna R, Soska R, Lun Y, et al. The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. Mol Ther. 2010;18:23–33.
Germain DP, Hughes DA, Nicholls K, et al. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med. 2016;375:545–555.
Hughes DA, Nicholls K, Shankar SP, et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet. 2017;54:288–296.
Beirao I, Cabrita A, Torres M, et al. Biomarkers and imaging findings of Anderson-Fabry Disease—what we know now. Diseases. 2017;5:15.
Maruyama H, Miyata K, Mikame M, et al. Effectiveness of plasma lyso-Gb3 as a biomarker for selecting high-risk patients with Fabry disease from multispecialty clinics for genetic analysis. Genet Med. 2019;21:44–52.
Niemann M, Rolfs A, Stork S, et al. Gene mutations versus clinically relevant phenotypes: lyso-Gb3 defines Fabry disease. Circ Cardiovasc Genet. 2014;7:8–16.
Nowak A, Mechtler TP, Hornemann T, et al. Genotype, phenotype and disease severity reflected by serum lyso-Gb3 levels in patients with Fabry disease. Mol Genet Metab. 2018;123:148–153.
Auray-Blais C, Lavoie P, Boutin M, et al. Biomarkers associated with clinical manifestations in Fabry disease patients with a late-onset cardiac variant mutation. Clin Chim Acta. 2017;466:185–193.
Yogasundaram H, Nikhanj A, Putko BN, et al. Elevated inflammatory plasma biomarkers in patients with Fabry disease: a critical link to heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7:e009098.
Weidemann F, Beer M, Kralewski M, Siwy J, Kampmann C. Early detection of organ involvement in Fabry disease by biomarker assessment in conjunction with LGE cardiac MRI: results from the SOPHIA study. Mol Genet Metab. 2019;126:169–182.
Arends M, Biegstraaten M, Hughes DA, et al. Retrospective study of long-term outcomes of enzyme replacement therapy in Fabry disease: analysis of prognostic factors. PLoS ONE. 2017;12:e0182379.
Franzen D, Haile SR, Kasper DC, et al. Pulmonary involvement in Fabry disease: effect of plasma globotriaosylsphingosine and time to initiation of enzyme replacement therapy. BMJ Open Respir Res. 2018;5:e000277.
Talbot A, Nicholls K, Fletcher JM, Fuller M. A simple method for quantification of plasma globotriaosylsphingosine: utility for Fabry disease. Mol Genet Metab. 2017;122:121–125.
US Department of Health and Human Services. Fabry disease: developing drugs for treatment. Guidance for industry. 2019. https://www.fda.gov/media/129690/download. Accessed 2020.
Barisoni L, Jennette JC, Colvin R, et al. Novel quantitative method to evaluate globotriaosylceramide inclusions in renal peritubular capillaries by virtual microscopy in patients with fabry disease. Arch Pathol Lab Med. 2012;136:816–824.
Gao F, Thompson P, Xiong C, Miller JP. Analyzing multivariate longitudinal data using SAS® 2006. Paper presented at: 31st Annual SAS® Users Group International Conference; March 26–29, 2006; San Francisco, CA.
Boutin M, Auray-Blais C. Multiplex tandem mass spectrometry analysis of novel plasma lyso-Gb(3)-related analogues in Fabry disease. Anal Chem. 2014;86:3476–3483.
Auray-Blais C, Boutin M. Novel gb(3) isoforms detected in urine of fabry disease patients: a metabolomic study. Curr Med Chem. 2012;19:3241–3252.
Sueoka H, Aoki M, Tsukimura T, Togawa T, Sakuraba H. Distributions of globotriaosylceramide isoforms, and globotriaosylsphingosine and its analogues in an α-galactosidase a knockout mouse, a model of Fabry disease. PLoS ONE. 2015;10:e0144958.
Quinta R, Rodrigues D, Assuncao M, et al. Reduced glucosylceramide in the mouse model of Fabry disease: correction by successful enzyme replacement therapy. Gene. 2014;536:97–104.
Germain DP, Waldek S, Banikazemi M, et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol. 2007;18:1547–1557.
Lenders M, Stypmann J, Duning T, Schmitz B, Brand SM, Brand E. Serum-mediated inhibition of enzyme replacement therapy in Fabry disease. J Am Soc Nephrol. 2016;27:256–264.
Mauer M, Sokolovskiy A, Barth JA, et al. Reduction of podocyte globotriaosylceramide content in adult male patients with Fabry disease with amenable GLA mutations following 6 months of migalastat treatment. J Med Genet. 2017;54:781–786.
Giugliani R, Waldek S, Germain DP, et al. A phase 2 study of migalastat hydrochloride in females with Fabry disease: selection of population, safety and pharmacodynamic effects. Mol Genet Metab. 2013;109:86–92.
Martineau T, Boutin M, Cote AM, Maranda B, Bichet DG, Auray-Blais C. Tandem mass spectrometry analysis of urinary podocalyxin and podocin in the investigation of podocyturia in women with preeclampsia and Fabry disease patients. Clin Chim Acta. 2019;495:67–75.
Doykov ID, Heywood WE, Nikolaenko V, et al. Rapid, proteomic urine assay for monitoring progressive organ disease in Fabry disease. J Med Genet. 2020;57:38–47.
Schiffmann R, Waldek S, Benigni A, Auray-Blais C. Biomarkers of Fabry disease nephropathy. Clin J Am Soc Nephrol. 2010;5:360–364.
Tanislav C, Guenduez D, Liebetrau C, et al. Cardiac troponin I: a valuable biomarker indicating the cardiac involvement in Fabry disease. PLoS ONE. 2016;11:e0157640.
Ortiz A, Germain DP, Desnick RJ, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123:416–427.
Acknowledgements
We thank Simon Heales, David Kasper, and Sarah Young for their contributions to the development of this study. Third-party medical writing assistance was provided by Lei Bai and Stephanie Agbu (ApotheCom, Yardley, PA)
Funding
This study was funded by Amicus Therapeutics, Inc.
Author information
Authors and Affiliations
Contributions
DGB, ABM, and NS participated in study design; DGB, ABM, NS, and EK analyzed the data; and DGB, CAB, HM, ABM, NS, and EK interpreted the data. JMA drafted the article. All authors critically revised the manuscript, gave final approval of the submitted version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Disclosure
D.G.B. has served as a consultant and speaker for, and received research funding and honoraria from, Amicus Therapeutics, Inc. and Sanofi Genzyme. J.M.A. has served as a consultant for Azafaros and as a speaker for Amicus Therapeutics, Inc. and Sanofi Genzyme. C.A.-B. has served as a consultant and speaker for Amicus Therapeutics, Inc. and Sanofi Genzyme; has served as a collaborator for 4D Molecular Therapeutics, Avrobio, and Protalix; has received research grants and honoraria as an investigator for BioMarin Pharmaceutical Inc., Shire/Takeda, and University Health Network; and has received research equipment and supplies from Waters Corporation. H.M. has received research support from Amicus Therapeutics, Inc. and Idorsia; and has received speaker fees from Amicus Therapeutics, Inc. and Sanofi K.K. A.T.B. has received honoraria from and served as a consultant and investigator for Amicus Therapeutics, Inc., Protalix/Pfizer, Sanofi Genzyme, and Shire/Takeda. N.S. is an employee of and holds stock in Amicus Therapeutics, Inc. E.K. is a paid consultant for Amicus Therapeutics, Inc. R.S. has served as a consultant for Chiesi Farmaceutici and Amicus Therapeutics, Inc., and has served as an investigator for Idorsia, Protalix, and Sanofi Genzyme.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author working as a consultant under the contract of Pharmaland Consulting Group.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bichet, D.G., Aerts, J.M., Auray-Blais, C. et al. Assessment of plasma lyso-Gb3 for clinical monitoring of treatment response in migalastat-treated patients with Fabry disease. Genet Med 23, 192–201 (2021). https://doi.org/10.1038/s41436-020-00968-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-00968-z
Key words
This article is cited by
-
A review and recommendations for oral chaperone therapy in adult patients with Fabry disease
Orphanet Journal of Rare Diseases (2024)
-
Circulating miR-184 is a potential predictive biomarker of cardiac damage in Anderson–Fabry disease
Cell Death & Disease (2021)
-
Pathology and pathogenic pathways in fabry nephropathy
Clinical and Experimental Nephrology (2021)