Abstract
Purpose
An entity of regression in Down syndrome (DS) exists that affects adolescents and young adults and differs from autism spectrum disorder and Alzheimer disease.
Methods
Since 2017, an international consortium of DS clinics assembled a database of patients with unexplained regression and age- and sex-matched controls. Standardized data on clinical symptoms and tiered medical evaluations were collected. Elements of the proposed definition of unexplained regression in DS were analyzed by paired comparisons between regression cases and matched controls.
Results
We identified 35 patients with DS and unexplained regression, with a mean age at regression of 17.5 years. Diagnostic features differed substantially between regression cases and matched controls (p < 0.001 for all but externalizing behaviors). Patients with regression had four times as many mental health concerns (p < 0.001), six times as many stressors (p < 0.001), and seven times as many depressive symptoms (p < 0.001). Tiered medical evaluation most often identified abnormalities in vitamin D 25-OH levels, polysomnograms, thyroid peroxidase antibodies, and celiac screens. Analysis of the subset of patients with nondiagnostic medical evaluations reinforced the proposed definition.
Conclusions
Our case–control evidence supports a proposed definition of unexplained regression in Down syndrome. Establishing this clinical definition supports future research and investigation of an underlying mechanism.
Similar content being viewed by others
INTRODUCTION
Interest and awareness of an unusual regression in some patients with Down syndrome (DS) have grown in the past decades, and 80 published cases from 9 previous studies guide us.1,2,3,4,5,6,7,8,9 In reporting and attempting to define this entity, clinicians have identified descriptive themes including loss of skills, mood changes, and repetitive thoughts or behaviors.1,2,3,4,5 These initial changes occur acutely or subacutely, oftentimes progressing over a range of months to years, in adolescents and young adults with DS; in published cases, the mean age of onset is 15.8 years, ranging from age 4 to 30.3,5,10 Some reports describe additional features such as psychosis, aggression, and catatonia.4 Presenting with changes in motor activity, unusual movements, changes in speech, and changes in oral intake, catatonia is uncommon in the general population with prevalence estimates ranging from 0.6% to 17% for children and adolescents and around 9% among subjects diagnosed with psychiatric or medical conditions.4,11 Catatonia, a distinctive diagnosis, which can be associated with psychiatric or organic causes, has been proposed by Judith Miles as an explanation for the functional decline in some of these patients with DS who are experiencing regression.4
Regression is also seen in patients with autism, a comorbid condition of up to 16% of individuals with DS, and in Alzheimer disease, a diagnosis reached by nearly 80% of individuals with DS who are older than 65 years.12,13,14 Nevertheless, studies have demonstrated key differences between regression in Down syndrome due to autism (mean age of onset of 62 months, male sex predominance,15 and lack of new-onset insomnia) and Alzheimer disease (lack of improvement, gradual rather than rapid decline without plateau, and later age of onset),10,15 versus the regression entity described here. Although overlapping features exist, regression in Down syndrome appears clinically distinguishable from other known diagnoses with loss of skills.
There is currently no consensus on the name of this entity within the clinical or scientific community. Several different diagnostic labels are currently being used with subtle differences: disintegrative syndrome,10 Down syndrome disintegrative disorder (DSDD),5,9 acute neuropsychiatric disorder,3 catatonia,2,4 regression,7 and acute regression.8 Based on DSM-IV criteria for autistic disorder, Gordon Worley has proposed the name DSDD, which stipulates that no other diagnoses may explain the condition.5,16 Similarly, catatonia not otherwise specified (NOS) is a unique diagnostic category included in the DSM-5 that could be considered in the absence of other psychopathology.4,17 One paper suggests that patients previously diagnosed with “acute regression” in the past could now be diagnosed with “catatonia NOS” based on DSM-5 criteria.8 Throughout this paper, we will use the clinically descriptive term “unexplained regression in Down syndrome” (URDS) and acknowledge that the name given to this regression entity may change as we learn more and clinicians reach more of a consensus.
At least five possible causes of URDS have been proposed: early changes associated with Alzheimer disease, disruption in routines or loss of support associated with the transition to adulthood, disruption in self-identity, inherent risk for catatonia, and autoimmunity.4,9,10 A clear pathophysiologic mechanism explaining why these causes only affect certain individuals with Down syndrome has not been identified and other causes, such as a new mental health disorder or untreated sleep apnea, may be suggested. Due to its unclear etiology, recommendations for medical evaluation, management, and treatment of URDS are limited. Abnormalities such as MRI changes, abnormal polysomnography, and increased incidence of thyroid autoimmunity in those with URDS have been reported.3,5,8 Although some studies have found no significant treatable causes, others have demonstrated success with low-dose psychotropic medications, electroconvulsive therapy, or immunotherapy.3,4,9
Despite this uncertainty in describing, naming, defining, evaluating, and treating URDS, it is clear that the condition has significant negative effects on the lives of the patient and family.10 The magnitude of these changes is significant: for example, one patient was described before regression as having “higher-than-expected adaptive functioning, including playing the piano, reading, and working part-time” but developed “progressive motor slowing, periodic cessation of all motor activity (freezing episodes), grimacing, posturing (a posture or movement maintained for a prolonged period), staring, loss of previously gained skills, markedly slowed food intake, negativism, and a loss of hedonic capacity” and was diagnosed with catatonia.4
A working group was created within the Down Syndrome Medical Interest Group (DSMIG-USA) to address the challenges of diagnosing and managing this entity.18 The working group created a definition consisting of 28 core and common clinical features of URDS. The goal of the definition was to create an operational/working definition that can be used by a clinician without need for special testing. It is criteria based and intended to have a severity threshold required to meet the definition (but without a specific preplanned number of criteria required). The domains were based upon expert opinion and observation of clinical cases by clinicians. The items were not based on specific questionnaires or instruments. Some of the members of the Regression Working Group are psychologists and developmental pediatricians, and had the opportunity to give input on the clinical features that were used to create the 28-item definition. The domains were then adopted by the workgroup to do additional case finding and study.
Core features include regression in adaptive function (change in functional activities of daily living [ADLs], speech, and social skills), cognitive–executive function (functional skills, declarative memory, procedural memory, learning memory, planning/organizing, and attention), and motor control (stereotyped movements, extrapyramidal, initiation–motivation, and catatonia); common features focus on behavior and mental health. A proposed, but previously unstudied, systematic approach to evaluating clinical deterioration including tiered medical evaluation was published by Jacobs et al.; these tiers include laboratory evaluation, imaging studies, psychiatry referrals, and other tests.6
We initiated this study to describe cases of URDS and to compare identified cases with age- and sex-matched control patients with DS. Regression cases from our multi-institutional, international DS database were compiled to answer the following clinical questions: (1) What are the consistent phenotypic features of URDS?; (2) What aspects of the clinical workup are most useful in patients with URDS?; and (3) How do our cases compare with those published in the medical literature?19 We hope that further refinement of the description of this entity will provide insight into the underlying cause of this type of regression and could inform the medical management of future patients with DS who develop similar symptoms.
MATERIALS AND METHODS
The literature review for this paper was conducted in PubMed using the terms “Down syndrome” and the various diagnoses given to this condition (“regression,” “Down syndrome disintegrative disorder,” “acute neuropsychiatric disorder,” “disintegrative syndrome,” “catatonia,” and “acute regression”); identified publications were reviewed and included if they contained original case data pertinent to this entity of regression that was not explained by another diagnosis.
An international consortium of DS clinics with a track record of clinical research and publication served as a pipeline for collecting clinical cases of URDS.19,20,21 Each site had an independent research team who consented its own patients, collected and maintained its own data in REDCap®, and possessed its own approval through the local ethics or institutional review board.22 The institutional review boards at Massachusetts General Hospital, University of Pittsburgh, University of Queensland, Mater Misericordiate Ltd, Duke University Medical Center, Nationwide Children’s Hospital, Ohio State University, and Bambino Gesu Children’s Hospital approved the study. We have received and archived written consent for participation/publication from every individual whose data are included. All information is presented in aggregate form. Consent for inclusion in the database was obtained during a visit to each site’s DS specialty clinic from all subjects.
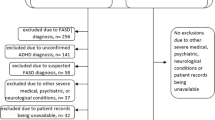
Beginning in 2017, sites collected a standard set of data through retrospective chart review. Inclusion criteria for regression cases included a clinical diagnosis of unexplained regression. As this is an entity without an established definition or clinical criteria, we used the clinical judgment of experienced physicians who provide medical care to individuals with DS in subspecialty clinics for DS to identify cases of unexplained regression. During monthly conference calls, physicians with cases of URDS presented the details of the patient’s presentation verbally describing past medical history, previous function, current function, physical exam findings, laboratory results, and imaging findings while physicians at other sites discussed the case as a group to decide if the patient should be included in this series. We vetted cases based on clinical presentation in comparison with the published literature rather than with the proposed diagnostic criteria. A group consensus was reached; if the group agreed that the case fit with URDS, the presenting physician then entered that patient’s standard set of data into the database.
Follow-up case review in September 2018 was conducted by physicians at each site to determine if new information had been learned, such as new diagnoses, new laboratory results, or treatment intervention with benefit, for any patients whom we initially included in our series. Exclusion criteria included a diagnosis of Alzheimer disease, autism spectrum disorder, or other diagnosis that accounted for regression symptoms (including cases whose symptoms at time of regression met criteria for, and were best explained by, a clinical diagnosis of major depression, psychosis, or other mental disorder). We excluded other causes of regression to focus our description on cases of unexplained regression in Down syndrome. (Of note, patients with autism could later develop unexplained regression but this was not the focus of our study.) One case was initially included but later excluded after symptoms improved spontaneously and suddenly without treatment, contrary to the clinical course for this entity as conceived. Inclusion criteria for controls were individuals with DS, consented in the database, who were first sex- and then age-matched (within 1 year), within each site.
The standard set of data collected for each subject included clinical details from (1) a definition of regression proposed by the chair of the Regression Working Group of the Down Syndrome Medical Interest Group and (2) a tiered medical evaluation.6 The 28-item proposed definition includes core and common clinical features based on frequency observed by clinicians familiar with URDS (defined in Table 2, and the checklist in the Supplementary Materials). Core features include regression in adaptive function, cognitive–executive function, and motor control; common features focus on behavior and mental health. For each subject, clinicians recorded if each core feature was present or absent with an onset of three months or greater.
The first tier of our medical evaluation included bloodwork (thyroid peroxidase antibody, thyroglobulin antibody, free thyroxine 4 [fT4], thyroid stimulating hormone [TSH], celiac screen, folate, vitamins D and B12, liver function tests, hemoglobin, white blood cell count, platelet count, electrolytes), imaging studies (abdominal radiograph, brain magnetic resonance image [MRI]), hearing and vision screens, and a polysomnogram. Apnea–hypopnea index (AHI) score as a measure of obstructive sleep apnea was recorded and annotated if treated or untreated. A screen for stressors and depression, focused on the six months prior to the onset of decline, was completed by the physician with parent input; we used a published, unvalidated DS depression screen.1 The second tier of medical evaluation included an electroencephalogram (EEG), antistreptolysin antibodies, antinuclear antibodies, Lyme antibodies, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rapid-plasma reagin, and HIV status. Laboratory values were recorded based on each institution’s normal range. All studies were not collected at all sites; patients who did not have a given evaluation completed were not included for that single parameter. In interpreting abnormal lab values, medical knowledge was used in deciding if abnormal values had clinical significance (e.g., an abnormal celiac screen was not counted as clinically significant if the patient had subsequently been transitioned to an appropriate gluten-free diet). The patient’s intelligence quotient (IQ) and age at assessment were recorded where available.
Standardized data sets were de-identified and compiled by a central statistician. Data were summarized using means and standard deviations, or percentages. To determine if demographic features, clinical symptoms, or abnormal medical evaluations in cases of URDS differed from matched controls, generalized mixed models were used for paired comparisons. Each model included a fixed case identifier and random effects of site and pair within site. Binary, ordinal, and continuous measures were modeled as Bernoulli, Poisson, and Gaussian random variables with a logit, log, and identify link function, respectively. This is equivalent to McNemar’s test for binary measures and a paired t-test for continuous measures with the addition of a random effect of site. Generalized mixed models yield a t-statistic testing each one degree of freedom contrast; p values result from comparing the estimated t-statistic for a given test against the central t-distribution. Subjects might not have completed all studies listed in the tiered evaluation, so a nondiagnostic medical evaluation refers to negative results on those tests that were completed. Of note, a nondiagnostic medical evaluation only refers to the medical evaluations that were completed and does not exclude medical causes that were not evaluated. To most stringently test our definition of unexplained regression and avoid confounding from unrecognized or untreated medical studies that could potentially contribute to regression, we repeated the analysis in the subset of patients with URDS who were free of any abnormal medical evaluation and their age- and sex-matched controls as a secondary analysis. Comparison-wise p values are reported for interpretation of the degree a given element differentiates regression cases and matched controls. Corrections for multiple comparisons were not applied as formal inference may not be applicable given the informal use of these same features by the consensus committee in assigning a given patient as a regression case or a control.
RESULTS
From 1 April 2017 to 30 September 2018, our six sites identified 35 patients (mean of 6 cases per site, range of 3–9) whose clinical symptoms fit with URDS among the patients who were enrolled in the consortium’s database. There were no significant differences between cases and controls on age, sex, ethnicity, and race (Table 1). Details of published cases, to date, identified through literature review and cases in our cohort were summarized (Supplemental Table). The 35 unique cases of URDS from our sites, of which 34 are previously unpublished, combined with the other 79 published cases, represent 114 total cases, to date (Supplemental Table). The age of onset of previously published cases (mean of 15.8 years, ranging from age 4 to 30 years) is similar to the age of onset in our cohort (mean of 17.5 years, ranging from age 9 to 34 years). The key clinical symptoms (motor, mood, and loss of skills regarding ADLs, speech, and social interaction) are similar to the previously published cases. Predominance among males1 or females8 has been seen; our cohort includes a nearly equal number of males and females with regression. Among all 114 cases, 60 (53%) were female, suggesting a higher incidence among females given the background 1:1.3 female-to-male sex ratio among individuals with DS (two-sided p = 0.06).
Patients with URDS showed consistency among the 28 clinical features included in the proposed definition. The four most common clinical features in URDS, present in more than 90% of our URDS cases, were changes in social skills (withdrawal, avoidance, isolation; time spent alone), functional ADLs (loss of acquired skills; dependent), attention (atypical, odd; gaze aversion, poor eye contact, or impaired ocular control), and internalizing behavior (apathy, withdrawal, mood, stereotype, self-injurious behavior). The least common clinical features, present in fewer than 10% of our URDS cases, include changes in autonomic symptoms (syncope, pallor, sweating), vision and hearing (acute loss or deterioration). Of the 28 core and common features of the proposed diagnostic criteria, cases with URDS displayed an average of 15.4 features (range 4 to 22).
The prevalence of diagnostic features differed substantially between cases with URDS and matched controls (p < 0.001 for all core features and for some common features; Table 2). Among core and common features, summed scores within each subgroup differed between cases with URDS and controls (Fig. 1). Among the three adaptive features, patients with URDS had a mean ( ± SD) of 2.7 ± 0.6 features while controls had 0.1 ± 0.4 features (t [34] = 6.21, p < 0.001). Of the six features related to cognitive–executive function, patients with URDS had a mean of 4.8 ± 1.6 features while controls had 0.1 ± 0.2 features (t [34] = 6.22, p < 0.001). Within the four motor features, cases with URDS had a mean of 2.4 ± 1.2 features while controls had none of these features. On the two behavior features, cases with URDS had a mean of 1.3 ± 0.5 features while controls had 0.2 ± 0.5 features (t [34] = 4.75, p < 0.001). Of the 13 mental health features, cases with URDS had a mean of 4.2 ± 2.0 features while controls had 0.9 ± 1.1 features (mean ratio = 4.45, 95% confidence interval [CI] 3.0 to 6.6, t [34] = 7.76, p < 0.001), driven by higher prevalence of issues related to mood, sleep, appetite, incontinence, and transition.
Within the first tier of medical evaluations, regression cases experienced more stressors and depressive symptoms. Cases with URDS had a mean of 1.1 ± 1.1 stressors while controls had 0.2 ± 0.4 stressors (t [34] = 4.27, p < 0.001), driven by higher prevalence of issues related to a change in school/workshop employment and loss of family member/friend/caregiver because of a move. Cases with URDS had a mean of 7.3 ± 6.7 symptoms on the depression screen while controls had 1.0 ± 2.3 (t [34] = 11.0, p < 0.001), driven by higher prevalence of issues related to insomnia, poor concentration, poor memory, loss of interest, social withdrawal, and a preference to be alone. The number of abnormal measures on tier 1 and tier 2 evaluation did not differ between cases with URDS and controls (t [34] = 2.32 and 0.80, p = 0.03 and 0.43, respectively). Among the medical evaluations completed, the studies most likely to be abnormal included polysomnography, vitamin D 25-OH level, celiac screen, and thyroid function tests (Table 3), but these were not statistically significant in comparison with the abnormal findings with controls. Thyroid peroxidase antibodies were abnormal in 8 of the 27 patients with URDS who had thyroid peroxidase (TPO) antibodies completed and in 2 of the 12 controls who had TPO antibodies completed. While some studies showed low yield, identified in only 1–2 individuals, many studies were not abnormal in any patients with URDS. Some patients were found to have multiple abnormalities on medical evaluation, with the number of abnormal studies ranging from 0 to 4 for patients with URDS. In total, 24 patients had at least one abnormality on medical evaluation. One component of the proposed tiered evaluation is screening for stressors and depression focused on symptoms prior to onset of regression; these differed between cases of URDS and controls (summary score, p < 0.001 and p < 0.001 respectively, Table 4).
The 11 patients with URDS with a negative medical evaluation had similar demographic characteristics to those of the total sample of 35 regression cases and to their matched controls. Among the subset of patients with a negative medical evaluation, the clinical features of the definition of URDS differed substantially between cases of URDS and matched controls (p < 0.05 for many measures, Table 2), similar to results seen in the total sample.
DISCUSSION
Unexplained regression in individuals with DS exists and differs from other entities in which regression has been described, such as autism and Alzheimer disease. Although identified as early as 1946, increased awareness and attention have occurred over the past decade regarding describing, naming, defining, evaluating, and treating this entity.4,5,6,8,23 To date, case reports have described individuals or cohorts, but none compared cases of regression with controls. This study is the first case–control study of URDS. We identified 35 cases of URDS at a mean age of 17.5 years, which were equally divided between sexes, with prevalence across races and ethnicities. We compared cases of URDS to age- and sex-matched controls on clinical features, depression and stressor screens, and medical evaluations. In this paper, 34 cases are previously unpublished, and 1 was previously published.6
In describing the consistent phenotypic features of this regression entity, we found that our patients with a clinical diagnosis of URDS matched well with the proposed diagnostic definition. The four most common clinical features were changes in social skills, functional ADLs, attention, and internalizing behavior. Our patients with URDS displayed an average of 15.4 of the 28 core and common features of the proposed diagnostic criteria while our control patients did not (average 1.3, range 0 to 6); cases with URDS did so across the proposed criteria with statistical significance for all measures except externalizing behavior. Our data support the proposed criteria and establish a definition for URDS.
Across the features studied, no single component identified all cases of URDS and simultaneously distinguished cases of URDS from controls. As such, the proposed multicomponent definition is essential to capture the essence of these cases. Patients with URDS did not have features isolated to a single category but rather had multiple features from multiple categories. No single feature was specific to URDS. For example, the clinical feature category of motor control was only seen in patients with URDS, not in controls, but was not seen in all cases with URDS (two patients with URDS did not have any of the four motor features). Conversely, control patients could occasionally demonstrate a single feature, such as hyperactivity, but did not demonstrate features across multiple categories. It is necessary to include items across features to capture all cases of URDS; we propose continued use of the 28-item proposed clinical definition as the established working definition of this entity. The 28 clinical features are summarized in the Supplementary Materials, which is formatted as a checklist tool for those considering this diagnosis. This checklist tool lists the same clinical features in the working definition, which were used in this study. The clinical features are followed by checkboxes for Present or Absent; the checklist proposes the following scoring system: total score (max 28): 0–3 unlikely URDS, 4–8 possible URDS, 9–15 probable URDS, 16+ highly likely URDS. A physician who identifies a patient with a score >4 may refer to a specialist with experience with this entity for additional guidance in diagnosis and treatment modalities. Drawing conclusions from this paper offers support for a clinical definition that will help others evaluating patients with features of URDS, will help unify clinical research to study this entity, and may help guide future research regarding its pathophysiology.
In investigating which aspects of the clinical workup are most useful, we studied the tiered medical evaluation that had been proposed in the literature among our patients with URDS.6 A tiered medical approach may be useful to guide clinicians who encounter patients with DS and features consistent with URDS in identifying contributing medical causes. Medical evaluation of our patients with URDS identified abnormalities, with higher yield for abnormalities found in tier 1 (65.7%) compared with tier 2 (42.9%). The studies with the highest likelihood of identifying an abnormality in patients with URDS included polysomnograms, vitamin D levels, celiac screens, and thyroid function tests. However, these abnormalities did not statistically differ among those seen in the matched controls. As such, these co-occurring conditions are likely not the sole explanations for URDS, but rather co-occurring conditions that might or might not exacerbate the regression.
The 11 patients with URDS with a completely negative medical evaluation were similar in demographics (Table 1), clinical features of URDS (Table 2), and stressor and depression screens (Table 4) to the total sample of patients with URDS. We conducted this analysis to ensure that co-occurring conditions were not impacting the use of the proposed diagnostic definition of 28 features. However, in doing this analysis, we also have important data regarding medical evaluation using the proposed tiered approach. We identified an increase in number of stressors and features of depression on these screens in patients with URDS compared with controls. This may be an important consideration for future study regarding risk factors and preventive screening to predict which patients may be at risk for URDS. Because the 11 patients with negative medical evaluation did not differ in definition, this raises the possibility that the tiered approach does not identify the underlying cause of regression. Future study should be conducted to evaluate the underlying cause given the clinical definition that we have established with attention to role of stressors and depression. We are hopeful that this definition can contribute to improving diagnosis, which can then be used to study this entity regarding the underlying mechanism, secondary genetic diagnoses, or modifier genes, and to describe future cases in a consistent way. Until we are able to more clearly elucidate the cause, continuing with this tiered approach to exclude other diagnoses that could contribute to symptoms might still be warranted. Future studies will be useful to investigate the contribution of these comorbidities to clinical symptoms as we follow patients longitudinally and study the outcome of treatment of co-occurring conditions.
The proposed first tier of medical evaluations, from Jacobs et al.,6 also includes a screen for stressors and depression screen focused on symptoms prior to onset of regression; these differed substantially between cases with URDS and control cases (summary score, p < 0.001 and p < 0.001 respectively). Differences in stressors and depression screen may provide insight into factors that precede the onset of symptoms. Future studies of the interplay among stressors, depression, and biology might identify an underlying predisposition to regression that requires additional psychological effects to manifest. If stressors and depression are found to precede regression, future studies following scores on these scales prospectively could be used to identify patients at higher risk for unexplained regression and to detect changes over time.
In comparing our cohort of cases of URDS with those published in the literature, we saw trends in similar age of onset, themes in symptomatology, and disease course. Adding our 34 new, unique regression cases to the 80 published cases substantially increases the number of reported cases. Our cohort of cases with URDS is the largest single cohort and the only to include case–controls in the literature to date. Our study systematically described cases in a consistent fashion using the proposed definition. As a team, our group identified the data elements to be collected a priori; each site collected data in a standard manner using the same terminology, timing, and data points. Although other studies have described cases of regression, our study adds to the literature through our systematic approach at testing this definition rather than relying on history and coding ex post facto. As a multisite study, our cases of URDS have greater generalizability than previous studies, which either focused on a single location or had a smaller sample size.
A multisite study design also creates potential for subtle differences between individual sites and physicians, which could be viewed as a limitation. However, cases of URDS were reviewed on monthly conference calls, and the common data dictionary of fields and responses in REDCap ensured that all databases were collected in a standardized way. The symptoms of URDS may change over time and overlap with psychiatric disease; for example, it may be difficult to recall when the depressive symptoms started in relation to the regression. A number of the associated features we have identified do occur in psychosis or depression. For example, the DSM-5 diagnosis of schizophrenia would only require catatonic behavior and negative symptoms (diminished emotional expression or avolition). Our single category of Mood, Emotion, Self-Regulation: Depression, Compulsions, Psychosis, PTSD, Anxiety, Panic, ASD/DSDD does not allow us to comment on the prevalence of psychotic symptoms within this group. In the future, a more detailed analysis of symptoms using a standardized and validated instrument could be considered to improve diagnostic accuracy. Additional potential limitations include that some data were entered retrospectively and thus subject to recall bias; not all patients had all evaluations completed in tiered workup, and thus our data were subject to potential information bias; and our patients, including cases with URDS and controls, were receiving care at DS subspecialty clinics with inherent selection bias, and thus may not generalize to the DS population overall. In the future, additional studies of the broader DS population could be conducted with this newly supported diagnostic definition for unexplained regression to replicate our findings. Additional factors to consider in the future include family history of mental illness and cognitive level prior to URDS.
Our results support the proposed definition of URDS, which consists of changes in multiple aspects of function. No single feature uniquely and distinctly identified URDS, supporting the need for inclusion of both core and common features in the definition. Tiered medical evaluation identified abnormalities, although these are of unclear significance to the underlying cause of URDS; patients with negative medical evaluation continued to fit with the proposed definition. Stressors and depression screen abnormalities were more prevalent in those with URDS, suggesting a psychological factor in this entity. With the supported definition, future studies can begin to focus on the nuances, causes, and ultimately treatment of URDS.
Conclusion
The 28-item proposed definition of URDS is supported by case–control evidence, providing a foundation for future research and investigation of underlying mechanisms.
References
Devenny D, Matthews A. Regression: atypical loss of attained functioning in children and adolescents with Down syndrome. In: Hodapp R, editor. International review of research in developmental disabilities. Vol. 41. San Diego, CA: Elsevier; 2011. p.233–264..
Jap SN, Ghaziuddin N. Catatonia among adolescents with Down syndrome: a review and 2 case reports. J ECT. 2011;27:334–337.
Akahoshi K, Matsuda H, Funahashi M, Hanaoka T, Suzuki Y. Acute neuropsychiatric disorders in adolescents and young adults with Down syndrome: Japanese case reports. Neuropsychiatr Dis Treat. 2012;8:339–345.
Ghaziuddin N, Nassiri A, Miles JH. Catatonia in Down syndrome; a treatable cause of regression. Neuropsychiatr Dis Treat. 2015;11:941–949.
Worley G, Crissman BG, Cadogan E, Milleson C, Adkins DW, Kishnani PS. Down syndrome disintegrative disorder: new-onset autistic regression, dementia, and insomnia in older children and adolescents with Down syndrome. J Child Neurol. 2015;30:1147–1152.
Jacobs J, Schwartz A, McDougle CJ, Skotko BG. Rapid clinical deterioration in an individual with Down syndrome. Am J Med Genet A. 2016;170:1899–1902.
Tamasaki A, Saito Y, Ueda R, et al. Effects of donepezil and serotonin reuptake inhibitor on acute regression during adolescence in Down syndrome. Brain Dev. 2016;38:113–117.
Mircher C, Cieuta-Walti C, Marey I, et al. Acute regression in young people with Down syndrome. Brain Sci. 2017;7:57.
Cardinale KM, Bocharnikov A, Hart SJ, et al. Immunotherapy in selected patients with Down syndrome disintegrative disorder. Dev Med Child Neurol. 2019;6:847–851.
Prasher V. Disintegrative syndrome in young adults. Ir J Psychol Med. 2002;19:101.
Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophr Bull. 2018;44:1133–1150.
Hithersay R, Hamburg S, Knight B, Strydom A. Cognitive decline and dementia in Down syndrome. Curr Opin Psychiatry. 2017;30:102–107.
Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–916.
McCarron M, McCallion P, Reilly E, Dunne P, Carroll R, Mulryan N. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome: 20-year longitudinal follow-up of dementia. J Intellect Disabil Res. 2017;61:843–852.
Castillo H, Patterson B, Hickey F, et al. Difference in age at regression in children with autism with and without Down syndrome. J Dev Behav Pediatr. 2008;29:89–93.
Bell CC. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA. 1994;272:828.
American Psychiatric Association, DSM-5 Task Force, American Psychiatric Association Publishing. Diagnostic and statistical manual of mental disorders: DSM-5, Arlington, VA; 2017.
Down Syndrome Medical Interest Group USA. https://www.dsmig-usa.org/. Accessed 4 January 2019.
Lavigne J, Sharr C, Ozonoff A, et al. National Down syndrome patient database: insights from the development of a multi-center registry study. Am J Med Genet A. 2015;167A:2520–2526.
Sharr C, Lavigne J, Elsharkawi IMA, et al. Detecting celiac disease in patients with Down syndrome. Am J Med Genet A. 2016;170:3098–3105.
Lavigne J, Sharr C, Elsharkawi I, et al. Thyroid dysfunction in patients with Down syndrome: results from a multi-institutional registry study. Am J Med Genet A. 2017;173:1539–1545.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
Rollin HR. Personality in mongolism with special reference to the incidence of catatonic psychosis. Am J Ment Defic. 1946;51:219–237.
Acknowledgements
Appreciation is given to the members of the DSMIG-USA Regression Working Group, whose efforts were essential to draw on unique clinical experiences, use expert opinion and observation of many cases by several MD or PhD clinicians, and collaborate to formulate the working definition of regression.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
B.G.S. occasionally consults on the topic of Down syndrome through Gerson Lehrman Group. He receives remuneration from Down syndrome nonprofit organizations for speaking engagements and associated travel expenses. He receives annual royalties from Woodbine House, Inc., for the publication of his book Fasten Your Seatbelt: A Crash Course on Down Syndrome for Brothers and Sisters. Within the past two years, he has received research funding from F. Hoffmann-La Roche, Inc. and LuMind IDSC Research Down Syndrome Foundation to conduct clinical trials for people with Down syndrome. He is occasionally asked to serve as an expert witness for legal cases where Down syndrome is discussed. He serves in a nonpaid capacity on the Honorary Board of Directors for the Massachusetts Down Syndrome Congress, the Board of Directors for the Band of Angels Foundation, and the Professional Advisory Committee for the National Center for Prenatal and Postnatal Down Syndrome Resources. He has a sister with Down syndrome. S.L.S. receives research funding from the LuMind IDSC Research Down Syndrome Foundation to conduct clinical trials for people with Down syndrome. She also serves on the Professional Advisory Board for the Massachusetts Down Syndrome Congress. C.F. receives research funding from Brain Injured Children’s Aftercare Recovery Endeavours (BICARE). She has a young nephew with Down syndrome. The other authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Santoro, S.L., Cannon, S., Capone, G. et al. Unexplained regression in Down syndrome: 35 cases from an international Down syndrome database. Genet Med 22, 767–776 (2020). https://doi.org/10.1038/s41436-019-0706-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-019-0706-8
Keywords
This article is cited by
-
Hypovitaminosis D in persons with Down syndrome and autism spectrum disorder
Journal of Neurodevelopmental Disorders (2023)
-
Immunotherapy responsiveness and risk of relapse in Down syndrome regression disorder
Translational Psychiatry (2023)
-
Evidence of neuroinflammation and immunotherapy responsiveness in individuals with down syndrome regression disorder
Journal of Neurodevelopmental Disorders (2022)