Abstract
Purpose
Access to genetics health-care services is often complicated by the distance to hospitals, workforce shortages, and insurance coverage. Despite technological advances and decreasing costs of genetic sequencing, the benefits of personalized medicine may be inaccessible to many patients. To assess potential disparities in care, we examined the genetics workforce in California and geographical issues that people encounter in seeking care.
Methods
Data on all board-certified genetics providers were analyzed including medical geneticists (MGs) and genetic counselors (GCs) in California. To assess distance traveled for care, we computed the distance patients traveled for n = 288 visits to University of California–San Francisco (UCSF) Medical Genetics. We performed geographic optimization to minimize the distance to genetics providers.
Results
The provider-to-patient ratio in California is 1:330,000 for MGs, 1:100,000 for GCs, and 1:1,520,000 for biochemical MGs. Genetics providers are concentrated in major metropolitan areas in California. People travel up to 386 miles for genetics care within the state (mean = 76.6 miles).
Conclusion
There are substantial geographic barriers to genetics care that could increase disparities. Our findings highlight a challenging genetics workforce shortage. The shortage may be even greater due to care subspecialization or lack of full-time equivalency and staffing. We are currently promoting efforts to increase remote health-care options, training, and modified models of care.
Similar content being viewed by others
INTRODUCTION
Genetic evaluations and testing have rapidly expanded into many areas of medicine as genomic tools have become more robust.1 Technological advances such as high-throughput sequencing have decreased the cost and contributed to a massive upsurge in genetic testing since the completion of the Human Genome Project.1,2 To illustrate, the number of sequenced exomes in one lab has increased from 3000 exomes in 2014 to 100,000 as of 2018.3
This rapid growth in technology has outpaced the growth of the genetics workforce. Previous medical genetics workforce studies have demonstrated an already critical shortage, which could increase in the coming years as genetic knowledge and clinical applications continue to expand.4,5 A workforce shortage could delay the widespread adoption of sequencing, because trained providers in medical genetics and genomics are essential for test interpretation and clinical care.
Despite rapid technological advances, the benefits of personalized medicine may be unequally distributed among patients. Access to genetics health-care services can be complicated by a number of factors including distance to medical centers, workforce shortages, and insurance coverage and networks.6
Previous groups have examined the genetics workforce in an effort to project supply and demand in the coming years.2,5,7,8,9,10 As genomic technologies are increasingly used in medicine, it becomes challenging to determine the demand for genetics services and establish a workforce shortage threshold. The Royal College of Physicians estimated a minimum requirement of 1:250,000 MG-to-patient ratio and the UK-based Association of Genetic Nurses and Counsellors set a threshold of 1:75,000–100,000 GC-to-patient ratio.2,11 Importantly, these estimates predate the advent of high-throughput sequencing.12
To assess the number of genetics providers and potential patterns in care, we examined the genetics workforce in California and the distances people traveled for in-person care. We hypothesize there is an overall workforce shortage and challenging distribution of providers in direct patient care. We also investigate the potential impact of the workforce shortage on vulnerable sociodemographic populations in California.
MATERIALS AND METHODS
Data on genetics providers in California
Data on board-certified genetics providers were extracted from the American Board of Medical Genetics and Genomics (ABMGG), the American Board of Genetic Counselors (ABGC), and the National Society of Genetic Counselors (NSGC). We compiled lists of medical geneticists (MGs), biochemical medical geneticists (BMGs), and genetic counselors (GCs) in the state of California. The list of MGs includes individuals with board certification in medical genetics and genomics. Each list was curated to confirm the primary practice locations and that providers were involved in direct patient care, which we also refer to as clinically active. Practice locations were then geocoded, meaning their geographical coordinates were calculated. Provider-per-population ratios were estimated using US Census Bureau population data and the curated list of clinically active MGs, BMGs, and GCs.
Minimum travel distance for genetics care in the state of California
We obtained a database of postal codes in the state of California from the US Postal Service and US Census Bureau data (n = 2654 postal codes). Postal codes were geocoded. Distance from each postal code to each provider was computed to find the minimum geospatial distance to a provider in California.
Clinical visits to genetics care
To assess the real distance that patients traveled for genetics care, we examined how far patients traveled for 288 consecutive outpatient visits to Medical Genetics at the University of California–San Francisco (UCSF). Ground travel distance was estimated from the centroid of each county to UCSF using Google Maps (https://www.google.com/maps). The shortest option for each county was selected in terms of both time and distance. International and out-of-state visits were excluded from the analysis. For a subset of consecutive visits (n = 111 of 288 visits), data including the type of insurance (private versus public) and ethnicity were also analyzed. The Urban–Rural Classification Scheme from the National Center for Health Statistics (NCHS) was used to group counties on an urban to rural scale.6 The six categories given by the NCHS Urban–Rural Classification Scheme were aggregated into three groups as follows: large central and fringe metro into large metropolitan, medium and small metro into small–medium metro, and micropolitan and noncore into micropolitan.
Statistical analyses
Descriptive statistics, geocoding, and creation of histograms and maps were performed with R software v.3.4.4 (https://www.r-project.org/), ggplot2 (http://ggplot2.org/), and ggmap (http://cran.r-project.org/web/packages/ggmap/).
RESULTS
Genetics providers in California
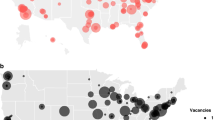
There are 871 board-certified genetics providers listed in the state of California including both MGs and GCs (MG = 197; GC = 674) (Fig. 1). Of these, 515 (59.1%) of providers are currently involved in direct patient care (MG = 119; GC = 396). Providers who are not directly involved in patient care per traditional hospital models work in other settings such as industry, public health, education, or are dedicated to research. A total of 26 providers (MG = 8, GC = 18) were employed in a different state. Industry employs 208/871 (24%) of the certified providers in the state. A small subset of 117 (13.4%) of providers do not have online presence, which may indicate they are currently retired or working in a different area. We identified 52 providers who are board certified in clinical biochemical genetics or medical biochemical genetics.7 Physicians comprised 41 of the 52 certified individuals, of whom 26 (63.4%) are currently clinically active. The additional boarded individuals (n = 11) have a PhD training background without an MD and are board certified in biochemical genetics working primarily in laboratory settings and subspecialize in inborn errors of metabolism.7 Twenty-one BMGs are also board certified as MGs.
Distribution and number of genetics providers in California. a Geocoded practice locations of providers in direct patient care (medical geneticists [MGs] in maroon and genetic counselors [GCs] in blue). b Total number of certified providers in California by genetics specialty, subset of the workforce dedicated to direct patient care (%), and estimated provider-to-patient ratio. BMG biochemical medical geneticists.
Considering only providers in active clinical practice, the provider-to-patient ratio in California is 1:330,000 for MGs, 1:100,000 for GCs, and 1:1,520,000 for BMGs, based on US Census data (Fig. 1). This provider-to-patient ratio assumes all providers have their time fully dedicated to clinical care; however, the actual clinical full-time equivalent (FTE) is <1. Providers in active practice are distributed across 44 institutions for MGs and 66 institutions for GCs. BMGs are distributed within 13 of these institutions. Providers are concentrated almost exclusively in larger metropolitan areas in the state. There is geographic overlap between the distribution of MGs and GCs (Fig. 1).
Minimum travel distance for genetics care in the state of California
As with a number of states and countries, California is challenged by care provision over geographical distance. By calculating the minimum travel distance from each postal code centroid to the nearest genetics provider in California, we were able to visualize areas that are very far from genetics providers, or “desert areas” (Fig. 2). The heat map demonstrates that individuals who reside outside large metropolitan areas need to travel farther for in-person genetics care. The average geospatial distance traveled from all postal codes is 34.2 miles, median 13.2 miles, and distances ranged up to 265 miles (Fig. 2b). Individuals from 22% of postal codes in California would have to travel a long distance to care (>50 miles) and individuals from 9.2% of postal codes would need to travel >100 miles to their nearest genetics provider (Fig. 2b).
Distance to genetics health-care services in California. a A heat map of the minimum geospatial distance between each state postal code centroid to its nearest genetics provider. Desert areas correspond to maroon-colored dots, located >50 miles away from genetics health-care services. b Distribution of theoretical minimum travel distances from all postal codes to the nearest genetics provider. c Geographic distribution of within-state visits to University of California–San Francisco (UCSF) (n = 282). d Real travel distance of visits to UCSF (n = 282).
Consecutive visits to UCSF Medical Genetics
To compare theoretical distances with real travel distance, we looked at n = 288 consecutive outpatient visits to UCSF Medical Genetics in San Francisco. People traveled up to 386 miles for genetics care within the state (mean = 76.6 miles, median = 59.0 miles) (Fig. 2d). Calculated travel time for visitors ranged up to 6.9 hours (mean = 1.63 hours, median = 1.31 hours). Geographic distribution of visits extended across 30 different counties of the 58 in California (Fig. 2c).
The average real travel distance was increased when compared with the theoretical minimum by 42.4 miles and the median distance increased by 45.8 miles. These data suggest that some people visited UCSF from counties that have a provider located closer to them.
To determine other factors that may be contributing to the increase in travel distance from the theoretical minimum, we examined demographic data of UCSF Medical Genetics patient visits (n = 111, Table S1). Sociodemographic data showed that non-Caucasian individuals were twice as likely to be publicly insured and that the ratio of publicly insured individuals increases as rurality increases. The median of the real travel distance was similar regardless of ethnicity (Fig. S1). Nonetheless, visitors with public insurance had a longer median travel distance than those with private insurance (median distance = 69.3 miles and 45.2 miles, respectively) (Fig. S1). The maximum travel distance increased by 92 miles for non-Caucasian individuals compared with Caucasians (Fig. S1).
DISCUSSION
California’s current genetics workforce supply of 1:330,000 for MGs and 1:100,000 for GCs is less than the recommended threshold according to previous workforce studies.2,5,7,8,9,11,12 Although the genetics workforce shortage is a known issue, we posit the workforce shortage to be even greater since previous surveys report that professionals allocate between 27.4% and 54.3% of their time to patient care.10,13 Providers often do not have a full 1 FTE since work time is distributed among other duties such as training the workforce, teaching, lab direction, administrative duties, and research.13 The estimated workforce shortage was marked for BMGs, with a provider-to-patient ratio of 1:1,520,000. Expansion of newborn screening programs to detect a growing number of treatable disorders and the possible inclusion of genomic sequencing to newborn screening may overwhelm the MG workforce. The growing demand for genetics providers in other health-care services, like testing companies, contributes further to the workforce shortage (Table S2).
Developing a US-based model to estimate the demand for the medical genetics workforce may be particularly difficult since the need for providers is dependent on numerous variables including access to services, awareness, referral patterns, and geographic location.2 With the advent of genomic sequencing and increasing knowledge in genetics, more conditions are being discovered. Genetics providers now care for a larger spectrum of patients including those with conditions that are not rare and conditions that are not “monogenic.”14 These trends will likely increase the workforce needs. The fact that medical genetics is a newer, developing field and that there is an ongoing discussion on the evolving roles of medical genetics providers only adds to the difficulty of calculating demand.2,8
However, in the United States, there is clear evidence for an increasing demand for genetic services and ongoing efforts to alleviate the workforce shortage.1,2,4,15 In late 2018, continued advocacy led to the 2019 Appropriations Bill, which permits a nationwide analysis of the medical genetics workforce.16 Through these efforts, the American College of Medical Genetics and Genomics (ACMG) and other entities hope to “more clearly identify workforce needs, implement programs and tools to fill workforce gaps and incentivize students to enter the field.”16 Several studies have also looked at innovating health-care delivery models, such as providing remote health care and helping primary health-care providers with the delivery of genetics services.17,18,19 Medical and laboratory genetics experts will also play key roles in facilitating care of a diverse population.
To gauge potential disparities in access to genetics care, we identified desert areas with minimal access to genetics services. Genetics services tend to be limited to large metropolitan areas. Genetics health-care deserts tend to be in smaller towns or rural centers, in the periphery of large cities. People who reside in rural areas of California have more limited access to genetics services due to distance. This limitation could lead to differences in health outcomes for underserved populations.
The average, median, and range of real distances that patients traveled are greater than the minimum theoretical distances calculated. People often travel farther than their nearest genetics provider. Factors like insurance limitations, provider subspecialization, wait times for appointments, and patient preference may contribute to the increase in travel distance. Our findings highlight a challenging geographic distribution of services in addition to a genetics workforce shortage. Regardless of insurance type or ethnicity, travel distances to genetics care in California are substantial. This may indicate that the genetics workforce shortage is so significant that it is impacting people of all backgrounds, regardless of their sociodemographic characteristics.
The data shows that 71.4% of people coming to UCSF Medical Genetics care from health-care deserts or micropolitan/rural areas are publicly insured. Publicly insured individuals have to travel a longer median distance by 24.1 miles compared with those with private insurance. It is possible that residents from more rural areas with public insurance are declined access to their closest center, thus creating a significant leap in distance to the next nearest genetics provider. Sociodemographic data from visits to UCSF Medical Genetics are consistent with data on health disparities, showing that minorities are more likely to be underinsured, a known social determinant of health.20,21 Despite these challenges to the health-care services, a high volume of visits at the center were provided to publicly insured individuals and minorities. Vast access to services can contribute to narrowing the existing gaps in health disparities.
Conclusions
In this study, we propose a model that identifies the distribution of genetic services and areas of need or desert areas. Distance can be used as an objective measure to help visualize the geographic barriers to genetics care that can lead to increased disparities. As groups continue to work on novel tools, models of delivery, training, and programs to alleviate the workforce gap, it is crucial to create models that integrate these advances and innovations for the population. We are currently promoting efforts to increase remote health-care options, education, and modified models of care.
References
Phillips KA, Deverka PA, Hooker GW, Douglas MP. Genetic test availability and spending: where are we now? Where are we going? Health Aff. 2018;37:710–716.
Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27:16–20.
GeneDx. https://www.genedx.com. 2000. Accessed 1 March 2019.
ACMG Board of Directors. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2015;17:505–507.
Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7:439–443.
Uribe-Leitz T, Esquivel MM, Garland NY, et al. Surgical deserts in California: an analysis of access to surgical care. J Surg Res. 2018;223:102–108.
Korf BR, Blitzer MG, Demmer LA, et al. Report on the Banbury Summit Meeting on medical genetics training in the genomic era, 23-26 February 2014. Genet Med. 2017;19:1–6.
Korf BR, Ledbetter D, Murray MF. Report of the Banbury Summit Meeting on the evolving role of the medical geneticist, February 12-14, 2006. Genet Med. 2008;10:502–507.
Korf BR, Feldman G, Wiesner GL. Report of Banbury Summit meeting on training of physicians in medical genetics, October 20-22, 2004. Genet Med. 2005;7:433–438.
Cooksey JA, Forte G, Flanagan PA, et al. The medical genetics workforce: an analysis of clinical geneticist subgroups. Genet Med. 2006;8:603–614.
Royal College of Physicians. Consultant Physicians Working with Patients The Duties, Responsibilities and Practice of Physicians in Medicine. Vol Revised 5t.; 2013.
Summar ML, Watson MS. LDTs, incidental findings, and the need for more geneticists. Medscape. 16 November 2015. https://www.medscape.com/viewarticle/853979_print. Accessed 14 August 2019.
American College of Medical Genetics and Genomics. 2017 salary survey report. August 2018. https://www.acmg.net//PDFLibrary/ACMG%202017%20Salary%20Survey%20Report%20August%202018.pdf. Accessed 14 August 2019.
Korf BR. Integration of genetics into medical practice. Growth Horm IGF Res. 2004;14:146–149.
Maiese DR, Keehn A, Lyon M, et al. Current conditions in medical genetics practice. Genet Med. 2019 Jan 28; https://doi.org/10.1038/s41436-018-0417-6 [Epub ahead of print].
Moran K. ACMG applauds increase in funding for newborn screening and medical genetics workforce study in FY 2019 LHHS appropriations. 15 October 2018. https://www.acmg.net/PDFLibrary/Statement_%20FY2019%20LHHS%20Appropriations_10.10_Final.pdf. Accessed 14 August 2019.
Elwyn G, Edwards A, Iredale R, et al. Identifying future models for delivering genetic services:a nominal group study in primary care. BMC Fam Pract. 2005;6:1–6.
Greendale K, Pyeritz RE. Empowering primary care health professionals in medical genetics: how soon? How fast? How far? Am J Med Genet. 2001;106:223–232.
Kubendran S, Sivamurthy S, Schaefer GB. A novel approach in pediatric telegenetic services: geneticist, pediatrician and genetic counselor team. Genet Med. 2017;19:1260–1267.
US Centers for Disease Control and Prevention (CDC). CDC health disparities and inequalities report—United States, 2013. 22 November 2013. https://www.cdc.gov/mmwr/pdf/other/su6203.pdf. Accessed 14 August 2019.
Fiscella K, Sanders MR. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health. 2016;37:375–394.
Acknowledgements
We thank ABMGG, ABGC and NSGC. We thank Colleen Carlston for her helpful suggestions. This research was supported in part by the Marcus Program in Precision Medicine Innovation at the University of California–San Francisco.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Penon-Portmann, M., Chang, J., Cheng, M. et al. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med 22, 227–231 (2020). https://doi.org/10.1038/s41436-019-0628-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-019-0628-5
Keywords
This article is cited by
-
The ENGAGE study: evaluation of a conversational virtual agent that provides tailored pre-test genetic education to cancer patients
Journal of Cancer Survivorship (2023)
-
A pilot study investigating feasibility of mainstreaming germline BRCA1 and BRCA2 testing in high-risk patients with breast and/or ovarian cancer in three tertiary Cancer Centres in Ireland
Familial Cancer (2023)
-
A systematic review of geographical inequities for accessing clinical genomic and genetic services for non-cancer related rare disease
European Journal of Human Genetics (2022)
-
An overview of genetic services delivery for hereditary breast cancer
Breast Cancer Research and Treatment (2022)
-
Investing in mentorship for our future patients’ needs
Pediatric Research (2021)