Abstract
Purpose
This study investigated whether genetic counseling and test reporting for the highly penetrant CDKN2A melanoma predisposition gene promoted decreases in sun exposure.
Methods
A prospective, nonequivalent control group design compared unaffected participants (N = 128, Mage = 35.24, 52% men) from (1) families known to carry a CDKN2A pathogenic variant, who received counseling about management recommendations and a positive or negative genetic test result and (2) no-test control families known not to carry a CDKN2A pathogenic variant, who received equivalent counseling based on their comparable family history. Changes in daily ultraviolet radiation (UVR) exposure (J/m2), skin pigmentation (melanin index), and sunburns between baseline and one year following counseling were compared among carriers (n = 32), noncarriers (n = 46), and no-test control participants (n = 50).
Results
Both carriers and no-test control participants exhibited a decrease one year later in daily UVR dose (B = −0.52, −0.33, p < 0.01). Only carriers exhibited a significant decrease in skin pigmentation at the wrist one year later (B = −0.11, p < 0.001), and both carriers and no-test control participants reported fewer sunburns than noncarriers (p < 0.05). Facial pigmentation did not change for any group. Noncarriers did not change on any measure of UVR exposure.
Conclusions
These findings support the clinical utility of disclosing CDKN2A test results and providing risk management education to high-risk individuals.
Similar content being viewed by others
INTRODUCTION
Due to substantial evidence that predictive genetic testing increases uptake of screening and prophylactic surgery,1,2,3,4,5 disclosure of genetic test results prior to disease onset is now recommended for predisposition variants for several cancer types, including breast and colorectal cancers. Predictive testing for the CDKN2A/p16 pathogenic variant, which confers a 28–76% lifetime risk of melanoma in the United States,6,7 may have similar benefits, but its clinical utility remains an open question. In contrast to other major hereditary cancer syndromes, familial melanoma risk management recommendations involve both accelerated screening and primary prevention behavior (i.e., avoidance of sun exposure). Although families with a history of melanoma indicate interest in CDKN2A testing,8 concerns have been raised that variant status does not substantially alter risk management recommendations.9,10 To support the clinical utility of CDKN2A testing, researchers must demonstrate that those who receive CDKN2A testing experience health benefits, such as improved risk-reducing behaviors, and have a low risk of adverse outcomes, such as a false sense of security among noncarriers.
The present study investigates whether disclosure of a CDKN2A pathogenic variant promotes reductions in sun exposure. Penetrance of CDKN2A is lower in regions with lower ambient ultraviolet radiation (UVR),7 suggesting that reductions in sun exposure may decrease melanoma risk among carriers. Likewise, sunburns and high-intensity UVR exposure during recreational activities—which are both common even among those with a family history of melanoma11,12—increase melanoma risk.13 Thus, reductions in sun exposure represent a clinically significant potential outcome of CDKN2A test disclosure.
To date, evidence about the benefits of predictive genetic testing for preventive behaviors for cancer and other diseases has been mixed. While some evidence indicates positive effects,14 other studies have not demonstrated a benefit of genetic testing on health-promoting behaviors,15 such as smoking cessation and physical activity. The only experimental study of the impact of genetic test reporting on risk-reduction behaviors for familial melanoma provided some initial evidence of benefit—those in a test-reporting condition (for CDKN2A or the less-penetrant MC1R) reported more recent skin self-examinations compared with those in a usual-care care condition, who received a mailed sun protection pamphlet.16 However, this study provided little information about the effects of genetic test disclosure on sun protection behavior because it included only five carriers. Further, risk management education was not equivalent across conditions, making it difficult to ascribe benefit to the test result itself rather than accompanying counseling.
Additionally, our previous prospective study of 59 members of melanoma-prone families suggested a long-term benefit of disclosing CDKN2A genetic test results. Specifically, unaffected carriers reported greater use of protective clothing and reduced sunburns 2 years following test reporting.17 However, like many studies of genetic counseling outcomes, this study lacked an equivalent control group that did not receive a genetic test result, and therefore could not distinguish the effects of test reporting from the effects of providing detailed education regarding melanoma risk. Further, like most studies of sun exposure,18 and all past studies of genetic testing for hereditary melanoma,11,15,16,19 the studies described above used self-reports of sun protection behavior, with little or no control for seasonality.
In the present study, we extend our prior research on behavioral outcomes of CDKN2A test reporting. We used a nonequivalent control group design to prospectively assess the impact of genetic test reporting both one month and one year later. The no-test control group was comprised of members of families with a strong history of melanoma, but no identified CDKN2A pathogenic variant, who received equivalent counseling about risk management but no genetic test (as none was appropriate for them). Because genetic test results are objective and highly personalized, we hypothesized that counseling accompanied by genetic testing would be more motivating than counseling based on family history alone. As a result, carriers would evidence lower daily UVR exposure and lighter skin pigmentation, and report fewer sunburns in the year following CDKN2A test reporting than no-test controls. This is consistent with our prior findings that carriers reported greater motivation for sun protection than no-test controls20 and greater understanding and acceptance of risk information and management recommendations.21
MATERIALS AND METHODS
The Utah Behavior, Risk Information, Genealogy, and Health Trial (BRIGHT) Project
Pre-established inclusion criteria, recruitment, and retention
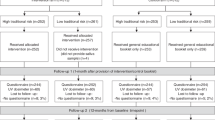
Participants ages 16–70 were recruited from melanoma-prone pedigrees of two types: pedigrees with an identified CDKN2A pathogenic variant, and pedigrees with a similar melanoma history but no identified CDKN2A pathogenic variant.22 Participants were excluded if they had received melanoma genetic counseling or had a melanoma history. Participants received $50 per visit and some received travel compensation. BRIGHT was approved by the University of Utah Institutional Review Board (IRB). All participants provided informed consent.
To determine appropriate sample size, a power calculation was conducted with the following parameters: power = 0.80, ɑ = 0.05, repeated-measures analysis of variance (ANOVA) with an effect size of 0.38, four timepoints, and three groups. Accounting for attrition of 25%, to test for main effects and one moderator, a minimum sample size of 28 was required for each group. Recruitment occurred during three spring-to-summer seasons from 2012 to 2014 to ensure that all sessions occurred during months with high UVR.23 Additionally, participants returned for follow-up visits 12 months after counseling, ensuring that follow-up assessments occurred at the same time of year (see Aspinwall et al.20 for additional details of recruitment procedure). Follow-up visits were completed one month and one year following genetic counseling.
Genetic counseling and test-reporting procedures
Individual genetic counseling was provided to all participants by one of two certified genetic counselors using a structured protocol,21 which was delivered with high fidelity (see Supplemental Materials). Members of CDKN2A+ families were provided pretest counseling to learn basic information about melanoma and genetic testing and provide genetic testing consent; they received results in a second session one month later. All genetic testing was performed in a CLIA-certified laboratory. No-test control participants were provided counseling only at their second visit because no genetic testing consent was applicable. For all participants, counseling sessions included a review of family medical history and education about melanoma risk factors, including environmental UVR and high-risk genes. Information provided to all participants about melanoma risk and its management was equivalent, except that members of CDKN2A+ families received a more specific risk estimate. Carriers were informed of their 70 in 100 lifetime risk for melanoma, while no-test controls were provided lifetime risk as a range from 30 in 100 to 70 in 100 due to family history, based on studies of families with a strong history of melanoma regardless of CDKN2A pathogenic variant status.24,25 Noncarriers were informed that they remained at an increased lifetime melanoma risk of 2 in 100 due to family history and other risk factors.26 All participants were instructed to reduce UVR exposure and perform monthly skin screenings.
Measures
Propensity scores
To improve our confidence that any differences in outcomes between study groups could be attributed to test disclosure, rather than potential pre-existing differences between the CDKN2A+ and no-test control families, propensity scores were calculated using 18 variables that might (1) differ between the two types of families at baseline, and/or (2) impact behavioral responses to genetic counseling. Significant differences were observed for just two of these variables (see Supplemental Materials). These scores, which conceptually represent likelihood of membership in a family known to carry the CDKN2A pathogenic variant, were entered as a covariate in all analyses.27 This approach allowed us to account for possible pre-existing differences on multiple variables while minimizing the number of covariates added to statistical models.
Daily UVR dose
As shown in the Supplemental Figure S1, UVR dose was assessed over three 27-day intervals by a Scienterra dosimeter.28 This wrist-worn battery-powered device assessed UVR dose at 10-second intervals. Participants were instructed to wear the device during all daylight waking hours. To ensure that the device would receive a comparable UVR dose to participants when they were engaged in water activities or otherwise determined that it would be inconvenient to wear the device, participants were asked to place the device in an area that received the same amount of sun as they expected to receive during the activity. The daily standard erythemal dose (SED; J/m2) was computed with software developed by the New Zealand National Institute of Water & Atmospheric Research29 and customized for this study. For individuals with fair skin, an SED of 2 corresponds to the minimal dose that can produce a slight reddening of the skin.30 For analyses, the device was considered “worn” for days on which there was a nonzero SED value.
Despite technical issues affecting one or more assessments among 28 participants and device loss or damage by 6 participants, dosimeter data were available for all attended study visits for 75.8% of participants. Of the 27 days of recording possible in each assessment period, the average number of days coded as “not worn” was 7.28 (SD = 6.48) at the counseling visit, 10.35 (SD = 8.61) at one month post-counseling, and 8.33 (SD = 12.87) at the one year follow-up visit; number of not-worn days did not differ by group. Multilevel model analyses retained all participants with a dosimeter data download for at least one assessment (n = 122).
Sunburn self-reports
At baseline, participants completed a single-item measure of past sunburns: “In the past 12 months, how many times did you have a red OR painful sunburn that lasted a day or more?”32 Response options ranged from 0 to 5 or more sunburns. At baseline, participants received a sunburn diary to record details of all instances of sunburn and their severity. To create a comparable metric between the survey and diary self-reports, reports of six or more sunburns in the diaries were recoded to 5. Prior to recoding the sunburn count from daily diaries, we observed two outliers – a 24-year-old male no-test control recorded 32; a 43-year-old male noncarrier, 10.
Skin pigmentation
A CR-400 Chroma Meter (Konica Minolta; Ramsey, NJ) assessed skin pigmentation via reflectance spectroscopy.31 The device captures wavelengths from 400 to 700 nm and measures the melanin absorption spectrum within this range, creating a unitless melanin index (MI) in which higher scores represent greater melanin content. At all four visits, readings were taken on four typically exposed sites (forehead, nose, cheek, dorsal wrist) and two typically unexposed sites (upper inner arm and lower back/buttock). At each site, five readings were averaged to enhance measurement reliability. MI scores of the forehead, nose, and cheek were averaged, forming a reliable facial composite scale (α = 0.81–0.91). To account for differences in natural skin color, the lowest-obtained MI score for the lower back/buttock was subtracted from each participant’s MI scores for exposed sites. Because artificial tanning products may inflate MI scores, data were excluded from reflectance analyses if these products were used within the last week. For reflectance spectroscopy, data were missing as follows (due to artificial tanner use or visit nonattendance): visit 1, wrist n = 6, face n = 3; visit 2, wrist n = 10, face n = 10; visit 3, wrist n = 14, face n = 10; visit 4, wrist n = 20, face n = 20. Multilevel model analyses retained all participants with skin pigmentation data for at least one assessment (n = 126).
Overview of analyses
Changes in daily UVR exposure, skin pigmentation, and sunburns were analyzed through multilevel modeling.33 This analytic method accounts for the nested structure of the data (i.e., assessments nested within individuals), uses maximum-likelihood procedures to account for missing data, and allows for inclusion of time-varying covariates (e.g., assessment date).33 To capture differences between participant groups, dummy-coded group variables were added representing the no-test controls and noncarriers, compared with carriers. Post hoc analyses examining group differences at the one-year follow-up are reported in the text. For multilevel modeling, the most critical assumption is independence of observations.33 Thus, to examine a potential dependency among members of the same kindred, we conducted multilevel analyses in which kindred (for all kindreds with five or more members) was included as a random effect. Inconsistent with the idea that family groupings contributed to model outcome, these models failed to converge for any measure of sun exposure and, therefore, were not reported.
RESULTS
As shown in Fig. 1, of 167 eligible participants offered study participation, 130 (77.8%) enrolled. Among the 37 who chose not to participate, the most common reasons listed were not interested/doesn’t want to participate (36.4% of those from CDKN2A+ families who declined, 33.3% of no-test control family members who declined), too busy (31.8%, 20%), and unwilling or unable to travel to the study site (9.1%, 6.7%). Two enrolled participants declined genetic testing and were excluded from analyses. Follow-up visits were completed one month (n = 124, 95.4%) and one year (n = 114, 87.7%) following genetic counseling. Attrition rates were equal across groups.
Sample characteristics
Nearly all (99.2%) of participants identified themselves as White (1.6% as Hispanic). The sample was 52% male, with an average age of 35.24 (SD = 13.99). Supplemental Table 1 displays additional demographic characteristics, which did not differ by group. Participants in this study came from 12 different kindreds. On average, 10.67 (SD = 12.22) participants came from each kindred, with this number ranging from 1 to 42. For CDKN2A cohorts, participants came from three kindreds, with 10, 26, and 42 members. No-test control families came from nine families, ranging in size from 1 to 16 members (M = 5.56, SD = 4.88).
Daily UVR dose
As shown in Fig. 2a, daily UVR dose at the initial assessment was higher among noncarriers (p = 0.03) than among no-test controls. The multilevel model analyses indicated that being male predicted a higher average daily UVR dose, while higher propensity scores predicted a lower average daily UVR dose (Table 1). As expected, daily UVR dose showed a curvilinear relationship with the date of assessment, such that UVR dose was highest during midsummer. As indicated by the significant negative coefficient for one month post-counseling, carriers showed a significant decrease from baseline in daily UVR dose. In contrast, UVR dose did not significantly change from baseline to one month among no-test control and noncarrier participants. Inspection of the assessment by group interactions indicated that the pattern of an immediate decline in daily UVR dose was unique to carriers.
At one year post-counseling, both carriers and no-test control participants showed a significant decrease from baseline in daily UVR dose. As indicated by the nonsignificant year by no-test control effect, this decrease in daily UVR dose was not significantly different between carriers and no-test control participants. For noncarriers, daily UVR dose did not change between baseline and either follow-up (p > 0.05). Analyses probing group differences in UVR dose revealed that at one year post-counseling, noncarriers had a significantly higher UVR dose than no-test controls (p = 0.007) and that UVR dose was not significantly different between carriers and noncarriers (p = 0.08).
Change in measures of sun exposure (UVR dose - a; Sunburns - b; Skin Pigmentation - c, d) from baseline through one year post-counseling among CDKN2A carriers, CDKN2A noncarriers, and no-test controls. CI confidence interval, MI melanin index, SED standard erythemal dose, UVR ultraviolet radiation. Change from baseline indicated by *p < 0.05, ^p < 0.10
Sunburns
The multilevel model analysis showed that older age and greater propensity scores predicted a lower number of sunburns (see Table 1). As shown by the year effect (and displayed in Fig. 2b), carriers did not report a significant change in number of sunburns between baseline and one year post-counseling, and the year by group interactions indicated no statistically significant differences in change over time between carriers and the other two groups. At one year post-counseling, carriers (p = 0.02) and no-test control participants (p = 0.02) reported fewer sunburns than noncarriers. Of note, most sunburns were rated as mild—only nine participants (7.3%; 3 from each group) recorded any severe sunburns.
Skin pigmentation
As shown in Table 1, assessment date exhibited the expected curvilinear relationship with MI scores. Those who were male and older also had higher MI scores. Higher skin type predicted higher MI scores for wrist values only. There was no significant change from baseline wrist and facial composite MI scores to the one-month follow-up for any group (Fig. 2c, d). Consistent with the prediction that reduction in sun exposure would be greatest among carriers, at the one-year follow-up, wrist MI scores had decreased significantly from baseline among carriers only. Further, as indicated by the significant group by year interactions, the slope corresponding to the decrease in wrist skin pigmentation between baseline and one year for carriers significantly differed from the slopes estimated for the other two groups. For the wrist, at one year only, MI scores were lower for carriers than noncarriers (p = 0.04), with no-test control participants intermediate to, and not significantly different from, carriers or noncarriers (p > 0.05).
Facial MI scores did not vary by group and did not significantly change following baseline. Facial MI scores were consistently lower than MI scores for the wrist, suggesting more consistent use of sun protection at this body site. For the face, no group differences were observed at any assessment.
DISCUSSION
Despite their increased risk, members of melanoma-prone families who have not developed melanoma nevertheless report high-intensity UVR exposure and sunburns.11,12 Predictive genetic testing can alert such individuals to elevated melanoma risk prior to disease onset, and thereby provide additional motivation for prevention behaviors. In the present study, we examined changes in sun exposure following genetic counseling and test reporting for the CDKN2A pathogenic variant, using objective and clinically meaningful measures. Analyses, which included rigorous controls for season and potential pre-existing group differences, indicated that individuals who received a positive CDKN2A genetic test result had reduced skin pigmentation at the wrist one year later and lower daily UVR dose starting the month following test reporting and sustained through the one-year follow-up.
The clinical utility of CDKN2A test reporting for unaffected family members has been debated due to concerns that variant status does not substantially alter risk management recommendations and could create adverse events, such as increased distress among carriers or a false sense of security among noncarriers.9,10 Contrary to these concerns, no studies of CDKN2A testing have found evidence of distress19,34 and we did not observe increases in sun exposure among noncarriers. Further supporting the clinical utility of CDKN2A testing, at one year post-counseling, carriers had lighter skin pigmentation and reported fewer sunburns than noncarriers.
In contrast to past studies evaluating behavioral outcomes of CDKN2A pathogenic variant testing, the present study included a no-test control group of individuals who had a comparable high risk of melanoma based on family history, but who were from kindreds in which a CDKN2A pathogenic variant had been ruled out as the cause of the family’s high rate of melanoma. Members of this group received identical risk management recommendations during their genetic counseling session (but no test result). Including this control group allowed us to identify whether and how genetic test disclosure promotes behavior change beyond the accompanying risk management education. In the present study, although both groups with highly elevated risk—carriers and no-test controls—showed decreased daily UVR dose at the one-year follow-up, analyses indicated a more immediate change among carriers. Importantly, the reflectance spectroscopy results supported a unique benefit of genetic testing: at the one-year follow-up, carriers alone exhibited decreases in wrist skin pigmentation. This pattern of findings suggests that while both no-test control participants and carriers took actions to reduce daily UVR dose (e.g., decreasing overall outdoor time, decreasing peak exposure time, and/or seeking shade), carriers may have additionally implemented improvements in sun protection during outdoor time (to which measures of skin pigmentation, but not UVR dose, are sensitive). Thus, research is needed to evaluate which specific behavioral changes underlie decreases in daily UVR dose and skin pigmentation, and whether the nature and extent/duration of these behavioral changes differ for those who receive a positive genetic test result.
Notably, disclosure of risk information was part of an intensive, clinic-based counseling and education session. Past research and theory indicate that risk messages are more effective when delivered with information about the specific actions needed to offset risk.35 Furthermore, behavioral improvements are more likely following risk communications that promote understanding of illness processes, such as the biological mechanisms through which a CDKN2A pathogenic variant and UVR exposure increase risk, as was done in the present study.36 Prior analysis of intermediate outcomes indicated that the counseling protocol used in this study improved understanding of melanoma among all groups.21 As the cost of genetic testing continues to decrease, making CDKN2A and other gene testing more accessible, it will be crucial to ensure that test disclosure is accompanied by effective education and counseling.
Strengths and limitations of the present study
Strengths of the present study include its recruitment of the largest number of unaffected carriers of CDKN2A yet examined and high retention of participants through the one-year follow-up. An additional strength is the use of objective measures of skin pigmentation and UVR exposure, compared with the self-report measures used in all other studies examining outcomes of CDKN2A test reporting. Further, we controlled for seasonality by ensuring all assessments occurred during months when the UV index averaged at least high (above 6), by controlling for assessment date in analyses, and by scheduling the one-year post-counseling visits to occur at the same time of year as the genetic counseling visit.
The primary study limitation was that random assignment was not used to control receipt of genetic test results. Withholding or delaying genetic testing presents ethical concerns given current professional guidelines for genetic counseling (as some family members would not receive the current standard of care)37,38 and CDKN2A testing.39,40 Therefore, we opted instead to recruit a no-test control sample with similarly high melanoma risk due to family history. Few differences were identified between participants from CDKN2A+ families and those from no-test control families, and propensity scores statistically controlled for multiple potential differences between the two kinds of families. CDKN2A+ families came from fewer kindreds than no-test control families, but our supplementary analyses did not identify an effect of kindred in our multilevel models. Despite the statistical and design controls for potential confounding variables, it remains possible that some other yet-to-be-identified factor(s) may account for the group differences reported here.
Although both recruitment and retention were high and did not differ between the two kinds of families, it is always possible that some form of selection bias may distinguish participants who elected to learn more about their cancer risk from those who did not (see Aspinwall et al.41 and Marteau et al.42 for discussion). In the present study, agreement to participate suggested a high degree of motivation among participants to learn more about their cancer risk. However, we note that reasons for nonparticipation in the two groups were similar and seemed unrelated to concerns about learning more about one’s melanoma risk.
An additional limitation of this study is that the sun exposure outcome measures varied in the extent to which they may have been impacted by the specific sun protection efforts taken on the part of the participant. For instance, UVR dose does not account for the concurrent use of sunscreen or protective clothing, while sunburns necessarily involve unprotected (or inadequately protected) UVR exposure. Likewise, the reflectance spectroscopy measure of skin pigmentation, while objective, may be impacted by variables in addition to recent sun exposure, including a participant’s natural skin tone (which was addressed by including clinician-rated skin type in multilevel models). A consideration of these measurement issues may elucidate why patterns of findings were not strictly equivalent across measures. For instance, although the MI data indicated a long-term reduction in skin pigmentation at the dorsal wrist among carriers, no corresponding reductions were found at the face. Even at baseline, facial MI values were markedly lower than those for the wrist, suggesting participants may have already engaged in greater sun protection of this body site. Because skin pigmentation is determined by various factors, including a variety of genes,43 large changes in sun exposure may be needed to produce measurable changes in skin pigmentation, particularly in individuals who do not tan well.
Conclusions and future directions
Genetic counseling about highly elevated melanoma risk, both with and without test reporting, led to sustained reductions in UVR exposure. Our results showed evidence of a unique benefit of test reporting, as carriers alone exhibited reduced daily UVR dose during the month following genetic counseling and lighter skin pigmentation at the wrist one year post-counseling. Importantly, no increases in UVR exposure were observed among noncarriers following a negative genetic test result. Future research might examine whether genetic counseling and testing for less-penetrant variants would similarly motivate long-term reductions in UVR exposure and whether these findings generalize to members of other cultural or ethnic groups. Investigations of the changes in sun protection behavior that influence UVR exposure, as well as specific barriers and facilitators to sun protection, are additional important steps toward understanding and improving responses to education about elevated melanoma risk.
References
Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol. 2009;28:510–518.
Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299:1320–1334.
Stoffel EM, Mercado RC, Kohlmann W, et al. Prevalence and predictors of appropriate colorectal cancer surveillance in Lynch syndrome. Am J Gastroenterol. 2010;105:1851.
Chai X, Friebel TM, Singer CF, et al. Use of risk-reducing surgeries in a prospective cohort of 1,499 BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2014;148:397–406.
Schwartz MD, Isaacs C, Graves KD, et al. Long term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer. 2012;118:510–517.
Begg CB, Orlow I, Hummer AJ, et al. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst. 2005;97:1507–1515.
Bishop DT, Demenais F, Goldstein AM, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J Natl Cancer Inst. 2002;94:894–903.
Taber JM, Aspinwall LG, Kohlmann W, Dow R, Leachman SA. Parental preferences for CDKN2A/p16 testing of minors. Genet Med. 2010;12:823–838.
Kefford RF, Mann GJ. Is there a role for genetic testing in patients with melanoma? Curr Opin Oncol. 2003;15:157–161.
Gerstenblith MR, Goldstein AM, Tucker MA, Fraser MC. Genetic testing for melanoma predisposition: current challenges. Cancer Nurs. 2007;30:454–461.
Bergenmar M, Brandberg Y. Sunbathing and sun protection behaviors and attitudes of young Swedish adults with hereditary risk for malignant melanoma. Cancer Nurs. 2001;24:341–350.
Aspinwall LG, Leaf SL, Kohlmann W, Dola ER, Leachman SA. Patterns of photoprotection following CDKN2A/p16 genetic test reporting and counseling. J Am Acad Dermatol. 2009;60:745–757.
Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60.
Frieser MJ, Wilson S, Vrieze S. Behavioral impact of return of genetic test results for complex disease: systematic review and meta-analysis. Health Psychol. 2018;37:1134–1144.
Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102.
Glanz K, Volpicelli K, Kanetsky PA, et al. Melanoma genetic testing, counseling, and adherence to skin cancer prevention and detection behaviors. Cancer Epidemiol Biomarkers Prev. 2013;22:607–614.
Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genet Med. 2014;16:846–853.
Perna FM, Dwyer LA, Tesauro G, et al. Research on skin cancer-related behaviors and outcomes in the NIH grant portfolio, 2000-2014: Skin Cancer Intervention Across the Cancer Control Continuum (SCI-3C). JAMA Dermatol. 2017;153:398–405.
Kasparian NA, Meiser B, Butow PN, Simpson JM, Mann GJ. Genetic testing for melanoma risk: a prospective cohort study of uptake and outcomes among Australian families. Genet Med. 2009;11:265–278.
Aspinwall LG, Stump TK, Taber JM, et al. Genetic test reporting of CDKN2A provides informational and motivational benefits for managing melanoma risk. Transl Behav Med. 2018;8:29–43.
Taber JM, Aspinwall LG, Stump TK, Kohlmann W, Champine M, Leachman SA. Genetic test reporting enhances understanding of risk information and acceptance of prevention recommendations compared to family history-based counseling alone. J Behav Med. 2015;38:740–753.
Eliason MJ, Larson AA, Florell SR, et al. Population-based prevalence of CDKN2A mutations in Utah melanoma families. J Invest Dermatol. 2006;126:660–666.
Climate Prediction Center. Daily UV index, Salt Lake City, UT, 2011. 2012. http://www.cpc.ncep.noaa.gov/products/stratosphere/uv_index/gif_files/slc_11.png.
Kefford RF, Newton Bishop JA, Bergman W, Tucker MA. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: a consensus statement of the Melanoma Genetics Consortium. J Clin Oncol. 1999;17:3245–3251.
Kefford RF, Salmon J, Shaw HM, Donald JA, McCarthy WH. Hereditary melanoma in Australia. Variable association with dysplastic nevi and absence of genetic linkage to chromosome 1p. Cancer Genet Cytogenet. 1991;51:45–55.
Hansen CB, Wadge LM, Lowstuter K, Boucher K, Leachman SA. Clinical germline genetic testing for melanoma. Lancet Oncol. 2004;5:314–319.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Allen M, McKenzie R. Enhanced UV exposure on a ski-field compared with exposures at sea level. Photochem Photobiol Sci. 2005;4:429–437.
Liley B, Liley J, Allen M, Robinson J, McKenzie R, Team U-VD. Personal exposures to UV radiation in New Zealand. Queensland, Australia: NIWA UV Workshop; 2010.
Gies P, Glanz K, O’Riordan D, Elliott T, Nehl E. Measured occupational solar UVR exposures of lifeguards in pool settings. Am J Ind Med. 2009;52:645–653.
Tuchin VV. Tissue optics: light scattering methods and instruments for medical diagnosis. Bellingham, WA: SPIE Press; 2007.
Glanz K, Yaroch AL, Dancel M, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008;144:217–222.
Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Thousand Oaks, CA: Sage; 2002.
Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Genetic testing for hereditary melanoma and pancreatic cancer: a longitudinal study of psychological outcome. Psycho-Oncology. 2013;22:276–289.
Witte K. Putting the fear back into fear appeals—the extended parallel process model. Commun Monogr. 1992;59:329–349.
Cameron LD, Marteau TM, Brown PM, Klein WM, Sherman KA. Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: the role of coherence. J Behav Med. 2012;35:286–298.
Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21:151–161.
Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70–87.
Leachman SA, Carucci J, Kohlmann W. et al. Selection criteria for genetic assessment of patients with familial melanoma. J Am Acad Dermatol. 2009;61:677.e1–e14.
Leachman SA, Lucero OM, Sampson JE, et al. Identification, genetic testing, and management of hereditary melanoma. Cancer Metastasis Rev. 2017;36:77–90.
Aspinwall LG, Taber JM, Kohlmann W, Leachman SA. Psychological aspects of hereditary cancer risk counseling and genetic testing. In: Carr BI, Steel J, (eds.) Psychological aspects of cancer. Boston, MA: Springer; 2013. p. 31–64.
Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C.et al. Effects of communicating DNA‐based disease risk estimates on risk‐reducing behaviours. Cochrane Database Syst Rev. 2010 Oct 6;10:CD007275.
Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18:R9–17.
Acknowledgements
This research was supported by the National Cancer Institute of the National Institutes of Health (R01 CA158322). Support was also received from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This research was also supported by the Huntsman Cancer Foundation (HCF); the Tom C. Mathews, Jr. Familial Melanoma Research Clinic endowment; the Pedigree and Population Resource of Huntsman Cancer Institute; and the Utah Population Database. This study also utilized the Utah Cancer Registry, which is funded by contract N01-PC-35141 from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program, with additional support from the Utah State Department of Health and the University of Utah. The authors acknowledge the use of the Genetic Counseling and Health Measurement and Survey Methods core facilities supported by the National Institutes of Health through National Cancer Institute Cancer Center Support grant 5P30CA420-14 awarded to the Huntsman Cancer Institute and additional support from the HCF. Partial salary support was also provided by Knight Cancer Institute and Oregon Health & Science University (S.A.L., P.B.C., and T.P.). T.K.S. acknowledges salary support by NIH/NCI training grant T32 CA193193 during the preparation of this manuscript. The authors additionally acknowledge the generous participation of all study participants who made this project possible. We thank also all members of the BRIGHT study staff for their contributions to the conduct or analysis of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
S.A.L. previously received an honorarium for her service on a Medical and Scientific Advisory Board for Myriad Genetics Laboratory and Castle Biosciences, Inc. She also collaborated with Myriad to test assays as part of an early access program that is unrelated to the present study. W.K. received a research grant from Myriad Genetics Laboratory to study the psychological and family communication outcomes of multigene panel testing. That project is unrelated to the present study. M.C. has served as a consultant for Invitae, a for-profit genetic information company, which is also unrelated to the study. The other authors declare no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stump, T.K., Aspinwall, L.G., Drummond, D.M. et al. CDKN2A testing and genetic counseling promote reductions in objectively measured sun exposure one year later. Genet Med 22, 26–34 (2020). https://doi.org/10.1038/s41436-019-0608-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-019-0608-9