Abstract
Purpose
Genome editing holds both tremendous therapeutic promise and significant potential risk. Sickle cell disease (SCD), the most commonly inherited blood disorder, is a frontline candidate for the clinical applications of this tool. However, there is limited knowledge of patient community values and concerns regarding this new technology. This study aims to investigate the perspectives of three key decision-makers (patients, parents, and physicians) toward participation in future CRISPR-mediated somatic genome editing clinical trials.
Methods
We utilized a mixed-methods approach, involving an educational video tool, two-part survey, and 15 moderated, audio-recorded focus groups, which were conducted in seven U.S. cities.
Results
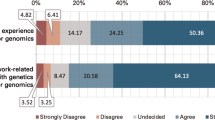
Study participants expressed hope that genome editing technology would rechart the course for SCD, but concerns related to involvement burden, uncertainty of clinical outcomes, and equity in access were identified. Major themes emerged from the focus groups: facilitators of, and barriers to, participation in future somatic genome editing clinical trials; information pertinent to the decision-making process; persons from whom participants would seek counsel before making a decision; and recommendations for the research community on meaningful engagement as clinical trials are designed and approved.
Conclusion
The advent of genome editing has renewed hope for the SCD community, but caution tempers this optimism.
Similar content being viewed by others

INTRODUCTION
One of the first targets of CRISPR-mediated somatic genome editing will likely be sickle cell disease (SCD, OMIM 603903).1,2,3,4,5,6 SCD affects millions of people, particularly those in regions where malaria is highly prevalent, such as sub-Saharan Africa, India, and the Mediterranean.7,8
SCD is caused by a single pathogenic variation (A→T) in the sixth codon of the β-globin gene. Affected individuals inherit two abnormal copies of the gene, resulting in the production of malformed hemoglobin. This diminishes the oxygen carrying capacity of erythrocytes, resulting in medical complications, including pain crises, strokes, pulmonary hypertension, leg ulcers, priapism, and acute chest syndrome.7,8,9
Despite being identified over a century ago and posing a significant global health burden, those living with SCD have limited treatments available to them.9,10 Hematopoietic stem cell transplantation (HSCT) remains the only nonexperimental cure for SCD.11,12 However, while the event-free survival rate of HSCT exceeds 90%, few patients can access this curative therapy due in part to stringent eligibility criteria.11,12 Further, while the life expectancy of the general adult SCD population has increased over the past 40 years, premature death continues.8,9
Because SCD is a well-studied molecular disorder impacting the blood system, it comprises an ideal candidate for gene editing therapies, with different approaches under current investigation. One mechanism involves promoting fetal hemoglobin (HbF) levels, which can reduce the disease’s severity by inhibiting HbS polymerization.5,7,13 However, HbF expression is typically suppressed after birth.13 Genome editing can be used to deactivate the B-cell lymphoma/leukemia 11A (BCL11A) transcription factor promoter, allowing HbF to persist.5,13 Other researchers have displayed proof of principle success in removing hematopoietic stem and progenitor cells (HSPC) from the bone marrow, correcting the pathogenic variation itself with CRISPR, and repopulating the bone marrow with the edited cells.2,4,14
Given these preliminary results, clinical trials are soon expected. On 13 September 2018, the National Heart, Lung, and Blood Institute launched the Cure Sickle Cell Initiative to accelerate the development of the most promising genetic-based curative therapies. However, given the fraught history of the SCD community’s medical marginalization, the community’s views must be a central consideration if the goal is to deliver successful, socially responsible research and health care.15,16,17,18 Michie and Allyse, in their study of parents of children with Down syndrome, concluded that genome editing interventions cannot succeed without input and support from patient communities.16 International policy positions have been enacted recommending stakeholder engagement, education, and bidirectional dialogue.17,19 “Publics must not only be asked to engage in the discussion, but they should also be given proper information and education regarding the known facts, as well as the uncertainties regarding the use of gene editing in research and in the clinic.”17 To this end, we sought to capture the perspectives of key stakeholders in the SCD community towards CRISPR-mediated somatic genome editing.
MATERIALS AND METHODS
Recruitment
Participants were recruited through collaborations with hematologists, community-based SCD organizations, and at national SCD conferences. Inclusion was limited to English-speaking adults. Eligible patients were required to have a diagnosis of SCD; parents had to have at least one child, pediatric or adult, diagnosed with SCD; and hematologists must have delivered care to at least five individuals living with SCD, pediatric or adult, for a minimum of 12 months.
Study design
Fifteen focus groups were conducted in the Southern and Mid-Atlantic regions of the United States between April 2017 and December 2017; these included six patient groups, six parent groups, and three physician groups (see Table S1). After providing informed consent and demographic data (Table 1), participants viewed a short educational video. The objective of the video was to provide participants with baseline scientific information about somatic genome editing and its potential use for SCD. The content of the video was reviewed by genomic researchers, genomic education specialists, and a science writer. Participants then answered survey questions related to genome editing and participation in future clinical trials. Focus group discussions followed. Trained moderators (A.P. and V.L.B.) led groups using a discussion guide, while another team member observed and took notes. Focus group questions were initially developed from topics identified through literature review and discussion. These questions were refined after the first three pilot groups. Each participant received a $75 gift card for their participation. This study was reviewed and approved by the Institutional Review Board at the National Human Genome Research Institute (NCT03167450).
Analysis
Debriefing sessions followed each focus group (A.P. and V.L.B.). An a priori list of codes, based on the focus group questions, was developed. These initial codes were modified, and other codes were added as needed to best capture the focus group data. Each code was defined. The interactions between participants and differences in opinion throughout the discussion topics were captured. Transcripts were independently reviewed by A.P. and S.D. using the qualitative analytic software NVIVO 11. Textual data were categorized using conventional content analysis techniques, as described by Shannon and Hsieh.20 Coded transcripts were compared, discrepancies were discussed, and intercoder reliability metrics were calculated. Discrepancies were resolved by re-examining the context of the quote within the transcript and returning to the original definitions assigned to the codes. The final kappa coefficient averaged 0.82, and percentage agreement scores of >90% were reached across all transcripts. Descriptive statistics were calculated for demographic variables and item-level attitudes toward genome editing and clinical trials.
RESULTS
Forty-six patients, 41 parents, and 23 hematologists participated in the study. Average age was 37.8 ± 12.6, 54.3 ± 9.6, and 53.6 ± 16, respectively. The majority of patients (88%) and parents (85%) self-identified as African American and/or Black. Forty-three percent of patients reported previous participation in clinical trials. Seventy percent of hematologists reported having previously conducted SCD research (See Table 1).
Four broad themes emerged from this work: (1) factors influencing one’s decision to participate, (2) information requirements for decision, (3) groups of individuals patients and parents would solicit guidance from, and (4) advice to the research community on meaningful engagement.
Decisional factors
Motivators
All three stakeholder groups were hopeful that gene editing could provide the overdue, impactful treatment for SCD many have been waiting for, often referencing the lack of treatments available compared with other diseases. Patients and parents discussed willingness to support future CRISPR-based clinical trials if suffering and social isolation are attenuated as a result (See Table 2)
:
“With me sitting here in pain right now…if there’s something that can be done to heal that, then I’m for it” (Patient). “I’m very optimistic. It’s another possible option for sickle cell patients and unfortunately we don’t have many” (Patient). Parents described the frustrating experience of seeing their children in pain, and feeling helpless to reduce the disease’s burden. Other patients and parents mentioned wishing they could have foreseen the toll SCD would take, or been given predictions of the trajectory of the disease’s severity:
“She can hardly breathe…the quality of life is just so horrible for them, and we have no control over it. As parents, we always want to fix things for our children, and we can’t…all I could do was get in bed with her, and hold her hand…and she’s 35” (Parent). “My son, he’s had five strokes…. As a young mother, would I have considered? Probably, if someone would have said that your child might avoid having strokes” (Parent).
For many patients, altruism was a salient motivator (see Table 2). Some were driven by the possibility that their participation could help family members living with the disease. Others saw participating as a way to promote social justice and support the SCD community at large. They felt participating might help reverse the lack of attention given to SCD, and encourage a more equitable distribution of resulting therapies:
“Because it’s a minority illness, it doesn’t get the consideration that it should. So, I would like to participate just so I can help somebody who’s coming behind me not to have it” (Patient).
Of patients reporting prior participation in clinical trials, 65% cited “helping others” as a primary incentive (see Table S2). In addition, 97% of patients indicated they would participate in a future CRISPR-based clinical trial to help other patients with SCD. Seventy-five percent said they would do so “for the sake of loved ones” (see Table 3).
Altruism surfaced far less among parents and was completely absent among physicians. Five times the number of parents and physicians cited lack of direct benefit to child or patient as a barrier compared with patients (see Table 3). However, while many parents viewed their child’s best interests as the main priority and expressed ambivalence over their child’s prospective involvement, a few recognized the value of research participation in accelerating treatment development and said they would let their child participate.
The perceived shortcomings of existing treatments, especially bone marrow transplantation (BMT), comprised another motivating factor (see Table 2):
“By age 10, I was in the hospital once a month. If I was given that decision, I probably would have said, ‘Mom, let’s do it’, but unfortunately, I was so unhealthy that I couldn’t go through with BMT” (Patient). “I can only speak for my one. There’s times where he’s just tired of taking pills…you got to take [it] every single morning, every single day” (Parent). “Is it like transplant and afterwards I have to take these pills the rest of my life?” (Patient).
Lastly, in two physician focus groups, there was mention of patients and parents being increasingly aware of, and willing to try, experimental treatments:
“They are wanting to know what the latest thing is- why can’t I have it? where are the trials?” (Physician)
Deterrents
Fear of participation stemmed from uncertainty about potential complications, the “editing genes” aspect of the CRISPR system, and the permanency of doing so (see Table 2). All three stakeholder groups were concerned about the unknown long-term effects of gene editing, a theme that surfaced in 12 of the 15 focus groups. Fifty-six percent of patients and 68% of parents cited “I don’t want to mess with [my/my child’s/my patient’s] genes” as a strong or moderate reason against participation, compared with 24% of physicians (see Table 3). Other patients and parents expressed anxiety over the possibility of exchanging one condition for another:
“Why is it so permanent? Once the DNA is cut and made, it can’t be undone, so that concerns me as well” (Patient).
Parents were afraid to make a decision that could potentially exacerbate their child’s disease severity. Issues around fertility and inheritance were raised. While these concerns surfaced among parents, they were absent from patient groups. Several parents wanted to avoid treatments that could limit their children’s reproductive viability, and a few were worried about possibly violating the fidelity of one’s family line.
“Are they going to be connected to me…as with my DNA? Are they going to be connected to my mother…and her mother and all of that?” (Parent)
The continued ability to pass down SCD was deemed a downside of somatic gene editing among patients and parents (see Table 2). Several participants asked about the complete eradication of SCD, mentioning the psychosocial implications of doing so, such as easier family planning. Physicians predicted this question and felt it was important to convey that somatic gene editing would not achieve this end.
All stakeholder groups noted the potential burden of trial involvement (see Table 2). These included questions regarding how many school days their child might miss, time off from work needed, possible relocation, the extent of follow-up, the length and nature of the recovery process, and its impact on family dynamics:
“We were considering a clinical trial and we had to go to Augusta and be there for two weeks, three weeks…that’s a lot. That’s a sacrifice to your family” (Parent).
Many patients and parents also expressed apprehension around the research enterprise’s trustworthiness and transparency (see Table 2). However, a few expressed confidence in the research process and regulatory bodies governing the enterprise. Several participants remarked that distrust could be mitigated by hearing from researchers who have committed their lives to helping those with SCD:
“I want to make sure that you don’t do this overnight just to get funding and put your name on something” (Patient). “I’m going to accept it more if it’s coming from my community…it has to be somebody who has been working to better this community before gene editing was a possibility” (Patient).
All three stakeholder groups worried about who would ultimately benefit (see Table 2). Many believed cost would be an issue in the future, and that those with the greatest need would have the least access:
“Are [we] going to be used to get whatever information…and then somebody else benefits from it [who] doesn’t even have the same disease?” (Patient).
Mediators
Religious beliefs, arising in eight groups, influenced decision-making in polarizing ways (see Table 2). Some participants viewed gene editing as “playing God” and inappropriately crossing a line, regardless of the goal, while others perceived it as a gift given by God to provide relief:
“From a spiritual perspective, I really disagree with it. At a DNA level, that’s how God intended you to be” (Patient). “I am fully supportive of using what God has given us to make our lives better” (Patient).
In 14 groups, the patient’s stage of life and capacity to manage the disease’s severity arose as another mediator (see Table 2):
“…in my 20s, it was hard. I think that if this came up in my 20s, my husband and I would have said ‘well maybe let’s try it’. I’ve been dealing with this for 45 years. I can deal with it for 45 more” (Patient).
Information needed to make decision
In addition to knowing the risks and benefits, participants cited three types of information pertinent to decision-making: clear specifics of the procedure and clinical expectations, interpretation of interpatient variation, and the track record of the research (see Table 2).
Patients and parents wanted to better understand the “cutting” and “repairing” aspects of CRISPR, details on the procedure itself, and contingency plans in the event the treatment goes awry. For many, reduction of pain was the central consideration. Others, however, inquired about improvements in other SCD comorbidities:
“And for those groups who have chronic iron overload, would that help us with our liver problems, would it help us with our avascular necrosis?” (Patient).
Physicians were particularly concerned by drawbacks of the procedure and urged communication of the limitations of gene editing:
“We change the genes in one of these patients, so they won’t have crisis but they’ve still got liver malfunction…all that stuff is still going to be there…. So, it’s not magic” (Physician).
All stakeholder groups wanted more details about the research supporting this type of treatment. Participants were interested in the length of the experiments, the duration of therapeutic effects, instances and causes of failures, on whom or what the experiments have been performed, and percentages of adverse events and successes:
“Show me every animal that died and why” (Patient). “I need to see that the red blood cells don’t go back to sickling…. Is it for a year and then it goes back?” (Patient). “Where are the current numbers on clipping [editing DNA] in the wrong place?” (Parent).
Lastly, participants asked how researchers are approaching the heterogeneity of the SCD population and determining eligibility criteria. All stakeholder groups thought it was important to determine conditions and critical windows for maximal effectiveness:
“Are success rates different for the different types of sickle cell?” (Patient). “Is this treatment going to be the last resort, everything failed, or would it be first resort, before anything wrong goes on?” (Physician).
Seeking guidance
Patients and parents cited five groups of people to consult before deciding to participate in clinical gene editing research: physicians, family members, other individuals with SCD who have previously participated in research, researchers, and religious leaders and/or God. Among potential advisors, trusted physicians with whom a long-standing, trusting relationship exists were identified as the group most patients and parents would consult. One parent, discussing his child’s physician, remarked:
“I think that relationships are extremely important, and when you build that trust with someone, they could tell you to ride this rocket ship to the moon, and you believe that they have your child’s best interests at heart” (Parent).
In addition, survey data revealed one-third of patients and parents previously participated in clinical trial research upon physician recommendation (see Table S2). Researchers, on the other hand, were least likely to be consulted. Patients and parents mentioned wanting to consult their physician two and a half more times across focus groups than they referenced research personnel:
“But you have people who did research and don’t want to give you the information. They just want you to participate” (Patient).
Recommendations for meaningful engagement
Each focus group was given an opportunity to leave the research community with some last thoughts on how to move forward (see Table 4). First, emphasis was placed on reaching out to the community to raise awareness and build credibility. Participants particularly stressed doing this sooner, rather than later. Many questioned why they were only hearing of CRISPR for the first time when the research has been ongoing for several years:
“Before you start saying, hey we’ve got this. Let me try this. Get the name out there more. Go to colleges, schools, urban communities, centers….” (Patient).
Across all groups, participants repeatedly mentioned giving SCD patients, parents, and advocates the chance to be actively involved throughout the entire research process; avoiding a one-size-fits-all approach, noting differences in culture and attitudes; and investing time to understand the lived experiences of SCD patients:
“I would want them to remember that they are doing this for real, live humans…these people have lives, families, and that this research should be conducted with care and consideration of those trying to benefit” (Patient). “I would say to listen. Don’t just ask us for opinions.” (Parent).
Patients and parents also wanted open access to information and complete transparency in the way this information is communicated. Patients, parents, and physicians urged that information be relayed through common communication modalities, specifically news channels, social media, talk shows, and other frequently used information distribution platforms:
“It should perhaps be set up a little bit different than what scientific communications have been before, which is all through these kinds of channels or journals or NYT science page. It should be on the talk shows and on more ordinary communication” (Physician). “Present it in a presentation just like it was presented to us…on a ground-level understanding” (Patient).
Finally, many participants noted injustice, often citing greater support given to other diseases with far lower rates of incidence. All stakeholder groups urged the research community to develop policies that promote equitable resource allocation and long-term access to novel treatments:
“To have the sickle cell population move this forward and then not have this available for them equally, would be extremely traumatic to the community” (Physician).
Physicians particularly stressed presenting the range of therapeutic options available both within and outside of the gene editing realm, as a way of avoiding inadvertent coercion and prioritizing patient interests. Emphasis was placed on clearly explaining the purpose of phase 1 clinical trials, and all possible implications of participation. Lastly, participants urged researchers to act in a manner sensitive to the fraught past between this patient community and the research enterprise:
“I think it is really important to also discuss what other cures or therapies may or may not be available….” (Physician). “I feel like we have one shot with this community. If things wane and we can’t maintain whatever is the production of the cure, then will they have something else that they can move forward with?” (Physician).
DISCUSSION
As the voices of disease communities grow louder and clinical trial development continues onward, the needs of the patients and families whose lives are likely to be altered by these new interventions must be prioritized.19 To our knowledge, this is the first study investigating SCD stakeholder views on somatic genome editing.
Despite long-standing claims that racial and ethnic minorities are less inclined to participate in clinical research, many participants in our study expressed excitement over this potential new treatment modality, but had needs and concerns they wanted addressed. An increasing number of studies suggest minorities are as willing as non-Hispanic whites to participate in clinical research.21,22,23,24 Research has shown there may even be an overrepresentation of minority communities in early phase clinical trials, when direct benefit is less likely.21 Physicians in our study also remarked that patients and parents in the SCD community often come to them seeking information about new experimental treatments. In 2014, Haywood and colleagues reported highly positive attitudes toward clinical trials among adults with SCD, with important facilitators being education, prior research participation, and perception of greater potential benefits.24 This study demonstrates a similar position towards somatic genome editing. Together, the mounting evidence against traditional theories of unwillingness to participate in clinical trials warrants a more nuanced examination of the barriers impeding enrollment.25,26
Patients, parents, and physicians also expressed fear of community exclusion from the long-term benefits of research. There was a pervasive concern that SCD patients might be used to help validate and improve the tool’s utility, after which profit-based incentives and efforts to treat other diseases would overshadow those who risked their lives to make these therapies a reality. Participants were also dissatisfied with how little they knew about gene editing prior to this study and felt that it was a key example of a gap needing attention. They proposed mechanisms of meaningful engagement they believed would be effective in building trust and increasing participation. These include partnering with advocacy organizations and trusted physicians and/or researchers, providing opportunities for patients and advocates to be actively involved, disseminating information about the current status of research via communication platforms frequently accessed by the community, and designing and supporting initiatives that promote the SCD community’s welfare. In an attempt to heed this advice ourselves, we have returned to the community and presented our findings since the conclusion of the study.
Our study also demonstrated that physicians were the group most participants would seek counsel from when deciding whether to participate in a CRISPR-based clinical trial. While patients and parents have often recounted bad experiences with clinicians in the emergency department, many mentioned having excellent, long-standing relationships with their hematologists. This suggests researchers should forge collaborations with these trusted physicians, be prepared to address their concerns, and work with them to better understand patient needs and establish rapport.27,28,29
This study had several limitations. First, 32% of patients and parents who self-identified as Black or African American reported some degree of college education. National census statistics estimate 8% of individuals with similar racial/ethnic backgrounds have obtained this level of education.30 Furthermore, most participants reported advocacy group engagement, which may not reflect the average SCD patient or parent. Our study population also appeared to be more actively involved in clinical trial research compared with the general SCD population, with 65% of our patients reporting having previously participated in a clinical trial. These attributes may restrict the generalizability of our findings to the broader SCD population. However, the research-engaged patient population are the patients more likely to participate in phase 1 gene editing clinical trials. Lastly, while the ability to draw conclusions from the quantitative data was limited by the small sample size, this data nevertheless informed and complemented focus group results.
The search for curative treatments using gene editing has renewed hope across the SCD community, providing a glimpse of a future with less pain, stigma, and neglect. However, there are cautionary, apprehensive undertones to this hope, partially due to the medical disenfranchisement of the SCD community. Using insights gained from this study and subsequent studies to inform the design and conduct of clinical trials will be crucial, especially with respect to consent and engagement. This exploration of SCD stakeholder views may also serve as a model through which to approach and understand the values of other patient communities, particularly those for whom CRISPR applications are currently being explored.
References
Bourzac K. Gene therapy: erasing sickle-cell disease. Nature. 2017;549:S28–S30.
Dever DP, Bak RO, Reinisch A, et al. CRISPR/Cas9 beta-globin gene targeting in human hematopoietic stem cells. Nature. 2016;539:384–389.
Canver MC, Orkin SH. Customizing the genome as therapy for the β-hemoglobinopathies. Blood. 2016;127:2536–2545.
DeWitt MA, Magis W, Bray NL, et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med. 2016;8:360ra134.
Canver MC, Smith EC, Sher F, et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527:192–197.
Collins F. NIH Director’s Blog. Sickle cell disease: gene-editing tools point to possible ultimate cure. October 25, 2016. https://directorsblog.nih.gov/2016/10/25/sickle-cell-disease-gene-editing-tools-point-to-possible-ultimate-cure/. Accessed 17 May 2018.
Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376:1561–1573.
Kato GJ, Piel FB, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010.
Lanzkron S, Carroll CP, Haywood C. Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public Health Rep. 2013;128:110–116.
Wailoo K. Sickle cell disease—a history of progress and peril. N Engl J Med. 2017;376:805–807.
Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–2317.
Bhatia M, Sheth S. Hematopoietic stem cell transplantation in sickle cell disease: patient selection and special considerations. J Blood Med. 2015;6:229–238.
Bauer DE, Kamran SC, Lessard S, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257.
Dever DP, Camarena J, Lee C, et al. Preclinical development of HBB gene correction in autologous hematopoietic stem and progenitor cells to treat severe sickle cell disease. Blood. 2017;130:4620.
ASH Clinical News. Legacy of medical racism could inhibit trials of CRISPR-based SCD treatments. March 5, 2018. https://www.ashclinicalnews.org/online-exclusive/trials-crispr-based-scd-treatments-must-contend-enduring-legacy-medical-racism/. Accessed 15 March 2018.
Michie M, Allyse M. Gene modification therapies: views of parents of people with Down syndrome. Genet Med. 2018;0:0.
Howard H, van El CG, Forzano F, Radojkovic D, Rial-Sebbag E, Borry P, De Wert G, Cornel MC. One small edit for humans, one giant edit for humankind? Points and questions to consider for a responsible way forward for gene editing in humans. Eur J Hum Genet. 2017;26:1–11.
Strong H, Mitchell MJ, Goldstein-Leever A, Shook L, Malik P, Crosby LE. Patient perspectives on gene transfer therapy for sickle cell disease. Adv Ther. 2017;34:2007–2021.
National Academies of Sciences, Engineering, and Medicine. Human genome editing: science, ethics, and governance. Washington, DC: The National Academies Press; 2017.
Hsieh H, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288.
Katz RV, Green BL, Kressin NR, Claudio C, Wang MQ, Russell SL. Willingness of minorities to participate in biomedical studies: confirmatory findings from a follow-up study using the Tuskegee Legacy Project questionnaire. J Natl Med Assoc. 2007;99:1052–1060.
Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health. 2011;101:2217–2222.
Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3:19.
Haywood C, Lanzkron S, Diener-West M, et al. Attitudes towards clinical trials among patients with sickle cell disease. Clin Trials. 2014;11:275–283.
Peters-Lawrence MH, Bell MC, Hsu LL, et al. Clinical trial implementation and recruitment: lessons learned from the early closure of a randomized clinical trial. Contemp Clin Trials. 2011;33:291–297.
Hughes TB, Varma VR, Pettigrew C, et al. African Americans and clinical research: evidence concerning barriers and facilitators to participation and recruitment recommendations. Gerontologist. 2017;57:348–358.
Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–476.
Sherber NS, Powe NR, Braunstein JB. Personal physicians as study investigators: impact on patients’ willingness to participate in clinical trials. Contemp Clin Trials. 2009;30:227–232.
Persaud A, Bonham VL. The role of the health care provider in building trust between patients and precision medicine research programs. Am J Bioeth. 2018;18:26–28.
United States Census Bureau. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Accessed 15 May 2018.
Acknowledgements
We would like to acknowledge all of the individuals, especially those living with SCD, who generously offered their time to participate in this study. This work was supported in part by the Division of Intramural Research, National Human Genome Research Institute (NHGRI) ZIAHG200394.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Persaud, A., Desine, S., Blizinsky, K. et al. A CRISPR focus on attitudes and beliefs toward somatic genome editing from stakeholders within the sickle cell disease community. Genet Med 21, 1726–1734 (2019). https://doi.org/10.1038/s41436-018-0409-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-018-0409-6
Keywords
This article is cited by
-
Decision-making about gene therapy in transfusion dependent thalassemia
BMC Pediatrics (2022)
-
States of Uncertainty, Risk–Benefit Assessment and Early Clinical Research: A Conceptual Investigation
Science and Engineering Ethics (2022)
-
Treatment decision-making in sickle cell disease patients
Journal of Community Genetics (2022)
-
How will new genetic technologies, such as gene editing, change reproductive decision-making? Views of high-risk couples
European Journal of Human Genetics (2021)
-
Disparities among infertility patients regarding genetic carrier screening, sex selection, and gene editing
Journal of Assisted Reproduction and Genetics (2021)