Abstract
Purpose
To estimate the prevalence of glucokinase variant-induced diabetes (GCK-DM) in a general population and to establish a clinical strategy for identifying GCK-DM from type 2 diabetes (T2DM).
Methods
A population-based study of diabetes in a rural region of Beijing, China, was conducted using two-stage stratified random cluster sampling. The glucokinase exons were sequenced in patients with diabetes.
Results
A total of 3345 subjects, including 545 patients with diabetes, participated in this study. Seven patients with GCK-DM were identified. The estimated prevalence rates of GCK-DM were 0.21% and 1.3% in the whole population and the diabetic patients, respectively. In the newly diagnosed diabetic patients (New-DM), a triglyceride cutoff ≤1.43 mmol/L (126.55 mg/dl) could discriminate GCK-DM from T2DM with 100% sensitivity and 68.4% specificity. Its effectiveness was confirmed in an additional 134 early-onset young patients with T2DM and mild hyperglycemia. A clinical criterion based on triglyceride and mild hyperglycemia could differentiate GCK-DM from T2DM in New-DM and was shown to be effective in identifying GCK-DM from 559 early-onset young patients with T2DM in the hospital.
Conclusions
The prevalence of GCK-DM is approximately 1.3% in the Chinese population with diabetes, and the new clinical screening strategy is helpful for identifying GCK-DM.
Similar content being viewed by others
Introduction
Maturity-onset diabetes of the young (MODY) is a form of autosomal dominant inherited diabetes. One benign subtype of MODY is caused by heterozygous inactivating variants in the GCK gene (GCK-DM)1,2,3 and is one of the most common forms of MODY in European Caucasians.4,5,6 To date, the prevalence of GCK-DM has been estimated in Caucasian pregnant women and Asian Indian children,7,8 and the prevalence of GCK variant-induced prediabetes (GCK-prediabetes) has never been evaluated. Because an increasing number of individuals with diabetes and prediabetes are diagnosed through the oral glucose tolerance test (OGTT) and hemoglobin A1c (HbA1c), the precise prevalence of GCK-DM and GCK-prediabetes in individuals identified through these diagnostic methods in the general population needs to be determined.
GCK-DM is characterized by mild asymptomatic hyperglycemia and a low risk of diabetic vascular complications,9,10 and it is sometimes falsely diagnosed as type 1 diabetes (T1DM) or type 2 diabetes (T2DM) and even receives inappropriate treatment. In fact, no treatment is needed for patients with GCK-DM, and hypoglycemic agents are often unnecessary and ineffective.11 With improvements in health care, more individuals are found incidentally to have asymptomatic hyperglycemia during health screenings; when they present to clinics for treatment, a correct diagnosis of GCK-DM is important to avoid incorrect treatment.
The current diagnostic testing for GCK-DM is DNA sequencing, but using this technique for every patient is not cost-effective, particularly in China, which is the country with the largest number of diabetic patients worldwide. Therefore, a more cost-effective strategy is needed to identify patients with GCK-DM in clinical practice. Although clinical screening criteria (CSCs) involving mild hyperglycemia (fasting plasma blood glucose [FPG] 5.4–8.3 mmol/L and HbA1c 5.8–7.6%) and 2-hour glucose increment during the OGTT (2h-GI) have been developed based on Caucasians12,13,14,15 for identifying patients for genetic testing, the applicability of these criteria to other ethnic groups has not been well evaluated. In this study, we aimed to estimate the prevalence of GCK-DM in a representative cohort randomly selected from the general population and to establish a clinical screening strategy to identify GCK-DM in Chinese population.
Materials and methods
Ethics statement
This study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at Peking University People’s Hospital. Written informed consent was obtained from all participants.
Study population
We conducted a population-based study of diabetes and metabolic syndrome in a rural region of China (Pinggu, located approximately 50 miles outside of Beijing) and established a cohort (Pinggu cohort). As previously described,16 this study was performed from March 2012 to May 2013 using two-stage stratified random cluster sampling; a total of 5004 residents aged 25–75 years were randomly selected, and 3345 subjects participated in the study (response rate: 66.95%), including 208 patients with previously diagnosed diabetes (PDM). Participants without a history of diabetes were given an OGTT. According to the American Diabetes Association (ADA) criteria for diabetes and prediabetes, 337 subjects were newly diagnosed with diabetes (New-DM) (FPG ≥7.0 mmol/L or 2-hour plasma glucose [2hPG] ≥11.1 mmol/L during the OGTT or HbA1c ≥6.5%), and 1438 subjects were prediabetic. A total of 545 patients with diabetes were included in this cohort (Table S1) and all of them were clinically diagnosed with T2DM because they had no other features of other types of diabetes such as T1DM (absolute insulin deficiency or insulin-dependent after the diabetes diagnosis), diseases of the exocrine pancreas and drug- or chemical- induced diabetes.
Biochemical measurements and clinical information
Blood samples were obtained in the morning after the patients had fasted for 8 hours. The HbA1c, creatinine (CRE), plasma glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglyceride (TG), and glycated albumin (GA) levels and the urinary albumin/creatinine ratio (ACR) were determined as previously described.16 Each subject underwent an ophthalmic examination using a TRC NW-8 fundus camera (Topcon Corporation, Tokyo, Japan). A homeostasis model assessment (HOMA) was used to evaluate β-cell function and insulin sensitivity.
Screening for variants in GCK exons and genetic analysis
The GCK exons and intron–exon boundaries were amplified and sequenced using the Sanger method. All the patients with diabetes in the Pinggu cohort were sequenced, the rare variants identified in this cohort were screened in a total of 500 normal subjects through Sanger sequencing, and family members of the patients with the rare variants were recruited. The following inclusion criteria were used for the normal control subjects: FPG <6.1 mmol/L, 2hPG <7.8 mmol/L during OGTT, HbA1c <6%, 45 years of age or older and no family history of diabetes. The pathogenicity of these variants were evaluated by the standards and guidelines recommended by the American College of Medical Genetics and Genomics (ACMG).17 We used two or more lines of computational evidence (PROVEAN [http://provean.jcvi.org], SIFT [http://sift.jcvi.org/], and PolyPhen-2 [http://genetics.bwh.harvard.edu/pph2/index.shtml]) to support a deleterious effect on the gene for PP3. Patients with pathogenic or likely pathogenic variants and mild hyperglycemia (FPG 5.4–8.3 mmol/L and HbA1c 5.8–7.6%) (ref. 14) were diagnosed with GCK-DM alone, and patients with pathogenic or likely pathogenic variants and clinical features of T2DM (severe hyperglycemia) were diagnosed with GCK-DM and concurrent T2DM.
Establishment of a new clinical screening strategy for a Chinese population
Based on the clinical features of GCK-DM identified in our study, we determined the cutoff values for the screening variables used for GCK-DM by analyzing the area under the receiver operating characteristic (ROC) curve (AUC), established new CSCs for GCK-DM according to our findings, and evaluated reported recommendations.12,13,14,15
Confirmation of the cutoff value in an independent group of early-onset young patients with T2DM and mild hyperglycemia and the evaluation of the new CSC performance in a clinical setting
Because mild hyperglycemia is widely accepted as a clinical screening method for GCK-DM,14 only a confirmation of the TGs cutoff was needed in patients with mild hyperglycemia. From January 2014 to April 2017, 559 patients with clinically diagnosed T2DM in our clinic who were younger than 45 years of age and were diagnosed before 40 years of age were included. Their clinical information was collected using the abovementioned methods. Their previous electronic hospital medical records were also evaluated. Subjects with typical clinical features of T1DM (absolute insulin deficiency [serum fasting C-peptide <0.6 ng/mL or undetectable] and insulin-dependent within 2 years after the diabetes diagnosis) or positive results of insulin, islet cell, and glutamic acid decarboxylase antibody tests were excluded. As shown in the flowchart (Fig. 1), a total of 134 patients with mild hyperglycemia at enrollment were screened for GCK variants to test the effectiveness of the TG cutoff.
Flowchart for distinguishing between patients with GCK-DM and patients with young early-onset clinically diagnosed type 2 diabetes in the hospital-based population. A total of 134 patients with mild hyperglycemia from among 559 patients clinically diagnosed with early-onset young type 2 diabetes (T2DM) who were younger than 45 years of age and diagnosed before 40 years of age were sequenced, and 3 cases of GCK-DM were detected. FPG fasting plasma glucose, TG triglycerides. HbA1c ≤7.6% (60 mmol/mol); FPG ≤8.3 mmol/L (150 mg/dl); TG ≤1.43 mmol/L (126.55 mg/dl).
Estimation of the prevalence of GCK-prediabetes in the Pinggu cohort
Because sequencing all prediabetic individuals would be expensive, only subjects meeting these CSCs from the 1438 prediabetic individuals in the Pinggu cohort were sequenced to identify carriers of the GCK variants and thus to estimate the prevalence of GCK-prediabetes.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS for Windows, version 23.0, Chicago, IL, USA). Normally distributed continuous variables are presented as the means and standard deviations (±SDs), and non-normally distributed variables are presented as the medians (interquartile range, IQR). Categorical variables are presented as numbers and percentages. Student’s t test or one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test was used to compare the means of quantitative traits, and either the X2 test or Fisher’s exact test was used for comparisons of qualitative traits. For non-normally distributed variables, the Kruskal–Wallis test was used. A P value <0.05 was considered significant.
We determined the ability of clinical variables to discriminate between GCK-DM and T2DM using the AUC. When the AUC was greater than 0.8, the clinical variable was considered useful for differentiating GCK-DM from T2DM.
Results
Identification of GCK-DM
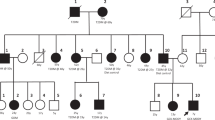
All variants identified in the 545 diabetic patients in the Pinggu cohort are shown in Table 1. Eight heterozygous missense variants were found in 11 individuals, including four variants (p.Arg43His, p.Gly44Ser, p.Arg377Leu, and p.Arg250Cys) reported to cause either GCK-DM or hyperglycemia.3,18,19,20 The clinical features of these 11 patients are summarized in Table 2. The members of three families (Figure S1) of probands with the p.Ala259Ser, p.Thr354Met, and p.Arg250Cys variants were recruited. The clinical features are shown in Table S2. In family 04, the proband and her son (04-02) had mild hyperglycemia and carried p.Ala259Ser, but the proband’s sister (04-03) had severe hyperglycemia and did not carry this variant, suggesting that she had T2DM. In family 06, three siblings (06-02, 06-03, and 06-04) of the proband carried p.Arg250Cys and presented with mild hyperglycemia. Because the proband (06-01) carried the same variant but had severe hyperglycemia, we diagnosed her with GCK-DM and concurrent T2DM. The proband’s younger sister (06-05) without this variant had impaired glucose tolerance but an HbA1c of 5.5%. Her HbA1c was outside the range of GCK-DM (HbA1c 5.8–7.6%) (ref. 13) In family 08, the proband of p.Thr354Met (08-01) had clinical features of T2DM and a history of severe hyperglycemia, which was similar to the other p.Thr354Met carrier (13-01) in the Pinggu cohort. The proband’s mother (08-05) and one sister (08-04) carried p.Thr354Met. However, they had normal OGTT results even though they were 85 and 58 years old, respectively; this finding does not support p.Thr354Met as a cause of hyperglycemia in this family, because the hyperglycemia of patients with GCK-DM is expected to be present from birth.14 Additionally, two carriers of p.Asp135Glu were identified in individuals with PDM in the Pinggu cohort. One p.Asp135Glu carrier was 65 years old and diagnosed with T2DM one year ago. She had a history of severe hyperglycemia and was treated with oral hypoglycemia agents initially; insulin treatment was added later due to poor glycemic control by oral agents alone. Another p.Asp135Glu carrier was 61 years old; he was treated with insulin for 16 years and had poor glucose control (HbA1c 9.3%) at the time of recruitment. All available information suggested that the p.Asp135Glu carriers had T2DM.
Briefly, among all the variants, three variants (p.Arg43His, p.Gly44Ser, and p.Arg250Cys) were pathogenic, three variants (p.Thr82Pro, p.Ala259Ser, and p.Arg377Leu) were likely pathogenic, and two variants were uncertain significance (p.Thr354Met and p.Asp135Glu) according to the ACMG (Table S3). Thus, we identified seven patients with GCK-DM from the Pinggu cohort (Figure S2); the prevalence rates were 2.1% in New-DM, 0% in PDM, and 1.3% in all the patients with diabetes. We extrapolated the prevalence of GCK-DM in the whole cohort to approximately 0.21%.
Clinical characteristics of GCK-DM in this study
Our study identified ten patients with GCK-DM, including seven (seven New-DM) from the Pinggu cohort (Table 2) and three (two New-DM and on PDM) from the probands’ families (Table S2 and Figure S2). Most patients (9/10) were diagnosed with diabetes through screening, and most (6/10) were obese or overweight according to Chinese-specific criteria.21 Three of the seven patients in the Pinggu cohort had a family history of diabetes. No patient with GCK-DM had retinopathy, but three of the ten patients had microalbuminuria or macroalbuminuria. Interestingly, three of the seven patients with GCK-DM who met the HbA1c-based criteria for diabetes were diagnosed with prediabetes according to the OGTT-based criteria.
All newly diagnosed patients with GCK-DM from the Pinggu cohort and the recruited family members were included in a study of the clinical features of GCK-DM. Patients with PDM were excluded from this analysis. The rationale for excluding the individuals with PDM was to develop a screening criterion using information reflecting the nature of the individuals with GCK-DM because the individuals with PDM were trying to control their diabetes. Significant differences in the serum TG, FPG, TC, LDL-c, and HDL-c levels (males) were observed between the patients with GCK-DM and the noncarriers of rare GCK variants (Table 3); however, the body mass index (BMI), blood pressure, HbA1c, and GA were similar.
Notably, only serum TG was discriminatory (Figure S3), with an AUC of 0.80 (95% confidence interval [CI] 0.71–89). The cutoff value for TG ≤1.43 mmol/L (126.55 mg/dl) was indicative of GCK-DM with 100% sensitivity and 64.8% specificity for all New-DM and 100% sensitivity and 68.4% specificity for the New-DM with mild hyperglycemia.
Establishment of a new clinical screening strategy for a Chinese population
Four clinical screening criteria (Table 4) based on previous recommendations14 and our findings were evaluated in the patients with New-DM from the Pinggu cohort, including CSC-1 (mild hyperglycemia and 2h-GI <4.6 mmol/L), CSC-2 (mild hyperglycemia), CSC-3 (mild hyperglycemia, 2h-GI <4.6 mmol/L and TG ≤1.43 mmol/L [126.55 mg/dl]), and CSC-4 (mild hyperglycemia and TG ≤1.43 mmol/L [126.55 mg/dl]). Higher sensitivity is necessary to increase the efficiency of screening for GCK-DM. The CSC-4 criterion, which showed 85.7% sensitivity and 83.6% specificity, was the most appropriate for differentiating GCK-DM from diabetes of other origins in individuals incidentally found to have mild hyperglycemia. In fact, CSC4 could picked up all patients with both GCK-DM and mild hyperglycemia in the Pinggu cohort.
Confirmation of both the TG cutoff and CSCs in a clinical setting
Among 134 early-onset young patients with T2DM and mild hyperglycemia, three patients (09-01, 10-01, and 11-01) were found to carry heterozygous variants (p.Tyr234His, p.Val412Glu, and p.Ser445Arg). These variants were not present in our controls and were pathogenic according to the ACMG (Table S3). The clinical characteristics of the probands and their family members are shown in Table S4. The proband with p.Val412Glu took metformin (1500 mg per day) for 1 year without any difference in the HbA1c levels before (6.8%) and after treatment (7.0%). After the patient was diagnosed with GCK-DM and stopped metformin, his HbA1c remained unchanged (6.9%) for the following 6 months. The proband with p.Ser445Arg was misdiagnosed with T1DM and treated with insulin for 4 years; however, his HbA1c levels (6.6–6.8%) demonstrated no significant changes.
Among the 134 patients with mild hyperglycemia (Fig. 1), a total of 69 patients with a TG level ≤1.43 mmol/L (126.55 mg/dl) at enrollment, including 3 patients with GCK-DM, were identified. The sensitivity and specificity of the ability of TG to distinguish between GCK-DM and T2DM were 100% and 49.6%, respectively. In contrast to the strategy based only on the measurements at enrollment (Fig. 1, path A), consideration of all previous hospital records (Fig. 1, path B) showed that only 25 subjects (8 patients took lipid-lowering agents, and 17 patients did not) had mild hyperglycemia in every record, and 7 patients (including 3 patients with GCK-DM) had TG levels that were lower than the cutoff in all hospital records. Thus, by sequencing 7 patients, we identified the 3 GCK-DM patients.
Estimation of the prevalence of GCK-prediabetes in a population with prediabetes in the Pinggu cohort
Among the 1438 prediabetic subjects in the Pinggu cohort, we identified and sequenced 207 subjects who met the CSC-4. Only one individual with a reported GCK variant (p. Gly318Arg) that was pathogenic based on the ACMG (Table S3) was identified.22 She was 32 years old, had a BMI of 23 kg/m2, 5.51 mmol/L FPG, 4.97 mmol/L PPG, 5.8% HbA1c, and 0.42 mmol/L TGs. Because CSC-4 could identify nearly all patients with GCK-DM and mild hyperglycemia in our study, we estimated that 0.07% of the prediabetic cases were due to GCK variants. Furthermore, among all sequenced subjects who met the CSC-4 criterion (207 prediabetic and 108 diabetic subjects) in the Pinggu cohort, the prevalence of causative GCK variants in the prediabetic subjects was lower than that in the diabetic patients (0.48% versus 5.6%, p = 0.007). Thus, we could also extrapolate that at least 0.24% of the cases with diabetes or prediabetes in the Pinggu cohort were caused by GCK variants. Given that some prediabetic subjects did not undergo sequencing, some cases with prediabetes caused by GCK variants may not have been discovered.
Discussion
For the first time, we estimated that the prevalence rates of GCK-DM or GCK-prediabetes in a cohort representing the general adult population were 0.24%, 0.07%, and 1.3% in the whole study and in the prediabetic and diabetic populations, respectively, although we might have underestimated the prevalence. The patients with GCK-DM exhibited mild hyperglycemia and lower TG levels compared with those in the patients with T2DM. The combination of FPG (5.4–8.3 mmol/L), HbA1c (5.8–7.6%), and TG levels (≤1.43 mmol/L [126.55 mg/dl]) yielded a higher sensitivity and specificity for discriminating GCK-DM from non-GCK-origin diabetes in New-DM and showed a better performance than did the reported recommendations based only on mild hyperglycemia. Based on multiple hospital records, our preliminary analysis also showed that in young subjects with clinically diagnosed T2DM characterized by mild hyperglycemia in a hospital-based diabetic population, a TG level less than 1.43 mmol/L (126.55 mg/dl) was both helpful and economical for identifying patients with GCK-DM.
Most prevalence studies of GCK-DM have been performed through the screening of subjects with mild hyperglycemia, but the precise prevalence of GCK-DM is unknown. GCK-DM is a common cause of incidental hyperglycemia in children23 but not in adults with mild fasting hyperglycemia, who represent approximately 0.76% of nondiabetic adults 30–70 years of age in hospital-based populations.12 Studies had estimated the prevalence of GCK-DM in specific subgroups of the general population.14 In one study,7 the prevalence of GCK-DM was calculated through screening a subset of patients from the population-based Atlantic Diabetes in Pregnancy study (n = 5500). The prevalence of approximately 0.11% of GCK-DM in the Caucasian female population was estimated.
In fact, we could roughly estimate the prevalence of GCK-DM in public databases, such as the Exome Aggregation Consortium (ExAC). We identified a total of 597 potentially causative variants of GCK-DM from the Human Gene Mutation Database (HGMD) and PubMed, among which 37 variants were present in ExAC. We reviewed and carefully evaluated all articles about these variants according to the ACMG and identified 23 of 37 variants that were pathogenic or likely pathogenic; thus, the corresponding percentage of GCK-DM in ExAC was 0.11% (Table S5). Because we could not determine whether some variants of GCK in the databases were associated with hyperglycemia according to available information, we might have underestimated its prevalence.
In contrast to previous studies, in our study, prediabetes and diabetes were classified using OGTT- and HbA1c-based criteria. Moreover, we estimated the prevalence of GCK-prediabetes using CSC-4. Although the TG cutoff was derived from the diabetic population, this strategy was unlikely to reduce the sensitivity of CSC-4 in identifying carriers of causative GCK variants in the prediabetic population because prediabetic individuals often have mildly abnormal lipid metabolism compared with that of patients with T2DM. Thus, we could have identified most causative GCK variants in the prediabetic population. Notably, fewer subjects carried causative GCK variants in the prediabetic population compared with the patients with T2DM among all sequenced subjects with mild hyperglycemia and TG levels less than ≤1.43 mmol/L (126.55 mg/dl), suggesting that the causative variants of GCK were more common in the diabetic population than in the prediabetic population. Additionally, it seems that GCK-DM is more common in Chinese than in Caucasians, and more patients with GCK-DM were found in the population with New-DM (2.1%) than in the PDM (0%). These findings are also explicable by the fact that we used both HbA1c and OGTT and identified more patients compared with only using OGTT. Further studies with larger sample sizes are needed to confirm these results.
Patients with GCK-DM may have gone undiagnosed for many years, which is explainable by our observations that patients with New-DM have an older age at diagnosis and lower FPG than do patients with PDM.
With the exception of one patient (06-01), who perhaps had concurrent T2DM, all the patients with GCK-DM presented with mild hyperglycemia. Microalbuminuria or macroalbuminuria occurred in three patients with GCK-DM, which might be attributed to concurrent hypertension; however, none of the patients with GCK-DM had retinopathy. Some patients with GCK-DM in this study had no family history of diabetes and presented with overweight and renal injury; thus, the BMI, family history of diabetes, and renal complications could not help precisely distinguish between patients with GCK-DM and T2DM.
In contrast to some (although not all) studies that have reported lower TG levels in patients with GCK-DM compared with healthy controls,24,25,26 only one study with a small size sample reported differences in TG between patients with GCK-DM and T2DM.27 We observed that patients with GCK-DM had relatively lower TG levels than did patients with T2DM; this finding can be explained by the fact that patients with T2DM often have hypertriglyceridemia, and a small sample size is sufficient to identify this difference between GCK-DM and T2DM. GCK controls glucose disposal and promotes glycogen and triglyceride synthesis;28 therefore, increased GCK activity could lower blood glucose concentrations at the cost of an increase in circulating triglyceride and free fatty acid (FFA) levels.29,30 Thus, heterozygous inactivating variants of GCK could explain the decreased triglycerides in patients with GCK-DM. In fact, some studies have also shown that common GCK variants are associated with the TG level.31 Additionally, the GCK regulatory protein (GCKR) can suppress activity of glucokinase,32 and several studies have consistently shown that its variants are positively associated with T2DM and FPG but are negatively associated with serum TGs.33 For example, the widely reported variant P446L of GCKR increases triglycerides and lowers glucose levels.34 These findings suggests that a relatively low TG level is a specific phenotype of carriers of pathogenic GCK variants.
Screening all patients for GCK variants is neither practical nor economical; therefore, some CSCs have been recommended to identify subjects for genetic testing. As shown in Table 4, both the 2h-GI and TG improved the specificity in patients with New-DM from the Pinggu cohort, but only CSC-4 had both high sensitivity and high specificity. In contrast to previous studies, we performed an association analysis between phenotype and genotype only in patients with New-DM without any intervention. Thus, we obtained a precise cutoff value for TG. Moreover, the effectiveness of the TG cutoff for the differentiation of patients with GCK-DM from T2DM with mild hyperglycemia was validated in 134 early-onset young patients with T2DM and mild hyperglycemia. We identified at least one patient with GCK-DM through genetic testing from 2.33 (3/7) subjects.
The CSC-4 would be economical for a correct diagnosis and individualized treatment for patients with GCK-DM, particularly in countries with a larger number of patients with diabetes and early-onset T2DM. However, the effectiveness of these criteria needs to be confirmed in other ethnic populations.
Importantly, the establishment of CSC-4 was based on mild hyperglycemia for the purpose of identifying GCK-DM individuals from patients with T2DM, which is the most common form of diabetes. In fact, patients with other types of diabetes, such as T1DM and other forms of MODY, usually present with severe hyperglycemia, which make their differentiation from GCK-DM easy.
Interestingly, in the Pinggu cohort, three of seven patients with GCK-DM who met the HbA1c criteria were diagnosed with prediabetes using the OGTT criteria, and the GCK-prediabetic patients who individually met the HbA1c criterion had normal OGTT findings. These findings suggest that the HbA1c criterion is more helpful than the OGTT criterion in screening for GCK-DM or GCK-prediabetes.
Previous studies have shown that GCK-DM is not common in East Asian populations with MODY or in early-onset patients with T2DM35,36,37 in contrast to Caucasians in developed countries,5,6 likely because most studied patients are recruited from hospital populations, and fewer patients with GCK-DM are included in these studies due to asymptomatic hyperglycemia. However, with improvements in health care, the number of subjects incidentally found to have mild hyperglycemia is increasing. Therefore, establishing a diagnosis of GCK-DM through genetic testing is important to avoid unnecessary treatment. Thus, patients with GCK-DM, particularly young patients, will benefit from our clinical screening strategies.
Our study has some limitations. First, not all the subjects in the Pinggu cohort were sequenced, and the prevalence of GCK variants in the whole cohort was merely estimated. Second, the pathogenicity of some variants of GCK identified in this study needs to be tested by the functional studies. Third, further studies with larger sample sizes are needed to replicate our findings.
In summary, the prevalence of GCK-DM is approximately 1.3% in the population with diabetes in China. The new criterion based on mild hyperglycemia and TG is helpful for identifying patients for genetic testing and individualized treatment.
References
Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345:971–980.
Matschinsky FM. Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes. 2002;51 Suppl 3:S394–404.
Osbak KK, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Human Mutat. 2009;30:1512–1526.
Chevre JC, et al. Mutation screening in 18 Caucasian families suggest the existence of other MODY genes. Diabetologia. 1998;41:1017–1023.
Massa O, et al. High prevalence of glucokinase mutations in Italian children with MODY. Influence on glucose tolerance, first-phase insulin response, insulin sensitivity and BMI. Diabetologia. 2001;44:898–905.
Feigerlova E, et al. Aetiological heterogeneity of asymptomatic hyperglycaemia in children and adolescents. Eur J Pediatr. 2006;165:446–452.
Chakera AJ, et al. The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: the Atlantic Diabetes in Pregnancy Cohort. Diabetes care. 2014;37:1230–1236.
Kanthimathi S, et al. Glucokinase gene mutations (MODY 2) in Asian Indians. Diabetes Technol Ther. 2014;16:180–185.
Steele AM, et al. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014;311:279–286.
Pruhova S, et al. Chronic mild hyperglycemia in GCK-MODY patients does not increase carotid intima-media thickness. Int J Endocrinol. 2013;2013:718254.
Stride A, et al. Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia. 2014;57:54–56.
Gloyn AL, et al. Prevalence of GCK mutations in individuals screened for fasting hyperglycaemia. Diabetologia. 2009;52:172–174.
Steele AM, et al. Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS ONE. 2013;8:e65326.
Chakera AJ, et al. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabetes Care. 2015;38:1383–1392.
Ellard S, Bellanné-Chantelot C, Hattersley AT, European Molecular Genetics Quality Network (EMQN) MODY group. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51:546–553.
Liu W, et al. Brain derived neurotrophic factor in newly diagnosed diabetes and prediabetes. Mol Cell Endocrinol. 2016;429:106–113.
Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424.
Gragnoli C1, et al. Early-onset type II diabetes mellitus in Italian families due to mutations in the genes encoding hepatic nuclear factor 1 alpha and glucokinase. Diabetologia. 2001;44:1326–1329.
Ziemssen F, Bellanne-Chantelot C, Osterhoff M, Schatz H, Pfeiffer AFTo, Lindner T, Cockburn BN, et al. Molecular genetics of MODY in Germany. Diabetologia. 1999;45:286–287. author reply 287-288 (2002).
Pinterova D, et al. Six novel mutations in the GCK gene in MODY patients. Clin Genet. 2007;71:95–96.
Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96.
Pruhova S, et al. Genetic epidemiology of MODY in the Czech Republic: new mutations in the MODY genes HNF-4 alpha, GCK and HNF-1 alpha. Diabetologia. 2003;46:291–295.
Yorifuji T, et al. Comprehensive molecular analysis of Japanese patients with pediatric-onset MODY-type diabetes mellitus. Pediatr Diabetes. 2012;13:26–32.
Berger M, et al. Are glucokinase mutations associated with low triglycerides? Clin Chem. 2005;51:791–793.
Tinto N, et al. Glucokinase gene mutations: structural and genotype-phenotype analyses in MODY children from South Italy. PLoS ONE. 2008;3:e1870.
Fendler W, et al. HDL cholesterol as a diagnostic tool for clinical differentiation of GCK-MODY from HNF1A-MODY and type 1 diabetes in children and young adults. Clin Endocrinol. 2011;75:321–327.
Spegel P, et al. Metabolite profiling reveals normal metabolic control in carriers of mutations in the glucokinase gene (MODY2). Diabetes. 2013;62:653–661.
Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27–42.
O’Doherty RM, et al. Differential metabolic effects of adenovirus-mediated glucokinase and hexokinase I overexpression in rat primary hepatocytes. J Biol Chem. 1996;271:20524–20530.
Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia. 2003;46:1662–1668.
Tam CH, et al. Interaction effect of genetic polymorphisms in glucokinase (GCK) and glucokinase regulatory protein (GCKR) on metabolic traits in healthy Chinese adults and adolescents. Diabetes. 2009;58:765–769.
Raimondo A, Rees MG, Gloyn AL. Glucokinase regulatory protein: complexity at the crossroads of triglyceride and glucose metabolism. Curr Opin Lipidol. 2015;26:88–95.
Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336.
Beer NL, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18:4081–4088.
Han XY, Ji LN. [Contribution of MODY2 gene to the pathogenesis of Chinese early onset familial type 2 diabetes]. Beijing Da Xue Xue Bao Yi Xue Ban. 2005;37:591–594.
Xu JY, et al. Genetic and clinical characteristics of maturity-onset diabetes of the young in Chinese patients. Eur J Hum Genet. 2005;13:422–427.
Hwang JS, Shin CH, Yang SW, Jung SY, Huh N. Genetic and clinical characteristics of Korean maturity-onset diabetes of the young (MODY) patients. Diabetes Res Clin Pract. 2006;74:75–81.
Acknowledgements
We thank the research staff Lianying Wang, Yu Zhu, Rui Zhang, Ling Chen, Xiuting Huang, Xueying Gao, and Fang Zhang, for the data collection. We also thank the study participants for their contributions. The Beijing Science and Technology Committee Fund (Z141100007414002) and the National Key Research and Development Program (2016YFC1304901) supported the DNA sequencing in this study, and the National High-Technology Research and Development Program of China (2012AA02A509) and the Beijing Science and Technology Committee Fund (D131100005313008) supported the data collection in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ma, Y., Han, X., Zhou, X. et al. A new clinical screening strategy and prevalence estimation for glucokinase variant-induced diabetes in an adult Chinese population. Genet Med 21, 939–947 (2019). https://doi.org/10.1038/s41436-018-0282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-018-0282-3
Keywords
This article is cited by
-
A comprehensive map of human glucokinase variant activity
Genome Biology (2023)
-
Insulin titration and blood glucose fluctuations during pregnancy in GCK-MODY: Case Report
International Journal of Diabetes in Developing Countries (2023)
-
Two novel GCK mutations in Chinese patients with maturity-onset diabetes of the young
Endocrine (2023)
-
The use of precision diagnostics for monogenic diabetes: a systematic review and expert opinion
Communications Medicine (2023)
-
Identification and management of GCK-MODY complicating pregnancy in Chinese patients with gestational diabetes
Molecular and Cellular Biochemistry (2022)