Abstract
Cystic fibrosis (CF) is one of the most common autosomal recessive life-limiting conditions affecting Caucasians. The resulting defect in the cystic fibrosis transmembrane conductance regulator protein (CFTR) results in defective chloride and bicarbonate secretion, as well as dysregulation of epithelial sodium channels (ENaC). These changes bring about defective mucociliary clearance, reduced airway surface liquid and an exaggerated proinflammatory response driven, in part, by infection. In this short article we explore the overlap in the pathophysiology of CF and COVID-19 infection and discuss how understanding the interaction between both diseases may shed light on future treatments.
Similar content being viewed by others
COVID-19 (SARS-CoV-2) infection triggers a cytokine storm, sepsis and life-threatening acute respiratory distress syndrome [1]. Patients with cystic fibrosis (CF) also manifest cytokine dysfunction and hyper-inflammation that overlaps with the pathophysiology of COVID-19 [2,3,4]. Intuitively, it might be concluded that CF patients infected with COVID-19 would be at high risk of serious illness. As a result, health services have responded with shielding or cocooning policies. Thus, a Mendelian randomised experiment is effectively underway, in real time, whereby patients with two mutant copies of the CFTR gene are being exposed to a new virus. While respiratory viruses, such as rhinoviruses and influenza, are associated with increased pulmonary exacerbations [5, 6], the morbidity and mortality from respiratory syncytial virus (RSV) infection is lower than expected in children with CF [7]. In a past epidemic of RSV, it was noted that relatively few patients with CF became severely ill. For example, at a time when so many babies became ill that a regional intensive care unit exceeded its ventilator capacity for sick children, not a single CF-affected child became ill (AM personal observations over two decades). This paucity of CF patients in the RSV cohort might be explained by the recent proposal that RSV may need an intact autophagic pathway for replication [8], allied to the finding that autophagy is dysregulated in CF cells [9]. There is some speculation that inducing autophagy, which is increased in CF, may counteract COVID-19 infection, although data remain limited [10].
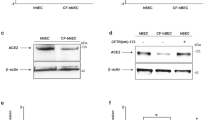
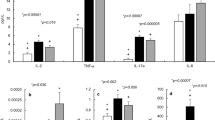
Conversely, there are sound theoretical reasons why CF might be expected to accentuate rather than mitigate the impact of COVID-19 infection. CFTR mutations disrupt cellular metabolism and exaggerate both lung and systemic inflammatory responses, with dysregulation of assembly of the multiprotein NLRP3 inflammasome complex that processes pro-inflammatory cytokines [2, 3] (Fig. 1). The SARS-CoV-2 virus enters host cells by using a spike protein to bind to the cell membrane protein, angiotensin-converting enzyme 2 (ACE2) [11, 12]. Cellular entry, via ACE2, is facilitated by the furin enzyme, making both critical players in infection. ACE2 has a site that is potentially activated by furin, which converts and activates viral surface glycoproteins and also regulates ENac [13]. Activation of furin, which is increased in CF [14, 15], together with the cellular damage induced by viroporins, might be expected to upregulate NLRP3 and cause inflammation [16]. We, and others, have reported that NLRP3 inflammasome is abnormal in CF cells [2, 3].
The spike protein on the virion binds to the ACE2 cell membrane protein. Cellular entry is facilitated by the TMPRSS2 and furin enzymes, one or more of which may be altered in CF. Once inside the cell, viral processing, may be influenced either by overactive inflammation and/or CF-affected cellular processes including autophagy, mitophagy, endosomal function and cellular metabolism, which may all be co-opted by COVID-19 for viral replication.
The role of furin in viral pathogenesis has recently been reviewed and the authors state that ‘the pathogenesis of some CoVs has been previously related to the presence of a furin-like cleavage site in the S-protein sequence’ [17]. For example, the insertion of a similar cleavage site in the infectious bronchitis virus (IBV) S-protein results in higher pathogenicity, pronounced neural symptoms and neurotropism in infected chickens. Thus, it is entirely plausible that furin activity may be a key factor in COVID-19 infections and the testing of furin inhibitors as therapeutic agents will be important in future studies [18]. The SARS-CoV-2 virus is reported to mimic the proteolytic activation of ENaC, an ion channel which is significantly upregulated in CF, where it drives inflammation and is critical to airway surface liquid homeostasis [19].
As yet there are limited data on the response of CF patients to COVID-19 infection, although preliminary information suggests that the course of disease may be milder than expected. Globally, from a population of about 100,000 patients, there have been over a hundred cases of COVID-19 infection in people with CF, with around 90% exhibiting relatively few symptoms and complications [20,21,22,23]. Although numbers and outcome may simply reflect effective shielding, it is highly likely that certain regions, such as New York State and Northern Italy, would have reported significant numbers of excess CF-COVID-19 deaths had patients been highly susceptible.
If further clinical experience indicates that the course of COVID-19 infection in CF patients is milder than anticipated, then it could be proposed that the relative protective effect associated with CF might accrue from CF-affected cellular processes linked to viral processing, including autophagy, mitophagy, endosomal function and cellular metabolism, which may all be co-opted by COVID-19 for viral replication [24, 25].
We hypothesise that CFTR modulator therapy might also confer additional benefit to patients with severe respiratory problems due to COVID-19 infection [2, 26]. For example, CFTR modulator therapy given to people with CF helps to restore cellular function, increases airway hydration, reduces oxidative stress, and down-regulates activation of the NLRP3 inflammasome [2]. The influence of CFTR in non-CF respiratory disease is intriguing and relatively poorly understood. Recent reports have demonstrated that acquired CFTR dysfunction occurs in smokers, and that the acute reduction in CFTR function due to cigarette smoke extract can be reversible by a CFTR potentiator in vitro [27, 28]. Carriers of the (commonest by far) Phe508del mutation found in over 70% of patients, have also been reported as having an increased risk of developing chronic bronchitis and bronchiectasis [29].
The role of CFTR in COVID-19 needs further elucidation in patients without CF. In an influenza model, the CFTR corrector, lumacaftor, was found to reverse in vitro down-regulation of CFTR and ENaC following viral infection and to restore airway surface liquid [30,31,32]. Both CFTR and ENaC have been proposed as theoretical cleavage sites for the coronavirus proteinase 3CLpro enzyme, which controls viral replication [33]. The transmembrane protease serine 2 (TMPRSS2), which can facilitate viral entry into the target host cell, also reduces ENaC activity in airway epithelium [34]. The detailed analysis of clinical outcomes in CF affected people may provide clues as to how these factors interact in the real world of COVID-19 disease.
The clinical importance of characterising the effects of COVID-19 infection in CF patients, and understanding the possible underlying protective effects, could shed light on novel targets and new approaches to antiviral therapy. We suggest that clinical trials of modern CF drugs should be explored in those infected by this new virus. In practice, a pragmatic trial is already underway, the outcome of which will depend on the response to COVID-19 in patients who either receive or do not receive modern CF drug combinations, and we also urge all CF registries to collect such case-control data to inform future studies.
References
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–13.
Jarosz-Griffiths HH, Scambler T, Wong CH, Lara-Reyna S, Holbrook J, Martinon F, et al. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife. 2020;9:e54556.
Scambler T, Jarosz-Griffiths HH, Lara-Reyna S, Pathak S, Wong C, Holbrook J, et al. ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. Elife. 2019;8:e49248.
McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;9:102537.
Flight WG, Bright-Thomas RJ, Tilston P, Mutton KJ, Guiver M, Morris J, et al. Incidence and clinical impact of respiratory viruses in adults with cystic fibrosis. Thorax. 2014;69:247–53.
Etherington C, Naseer R, Conway SP, Whitaker P, Denton M, Peckham DG. The role of respiratory viruses in adult patients with cystic fibrosis receiving intravenous antibiotics for a pulmonary exacerbation. J Cyst Fibros. 2014;13:49–55.
Eymery M, Morfin F, Doleans-Jordheim A, Perceval M, Ohlmann C, Mainguy C, et al. Viral respiratory tract infections in young children with cystic fibrosis: a prospective full-year seasonal study. Virol J. 2019;16:111.
Zhao Y, Li Z, Zhang L, Lian H, Ma H, Wang D, et al. Clinical features and outcomes of patients with hemophagocytic lymphohistiocytosis at onset of systemic autoinflammatory disorder and compare with Epstein-Barr virus (EBV)-related hemophagocytic lymphohistiocytosis. Med (Baltim). 2020;99:e18503.
Maiuri L, Raia V, Piacentini M, Tosco A, Villella VR, Kroemer G. Cystic fibrosis transmembrane conductance regulator (CFTR) and autophagy: hereditary defects in cystic fibrosis. Oncotarget. 2019;10:4492–500.
Carmona-Gutierrez D, Bauer MA, Zimmermann A, Kainz K, Hofer SJ, Kroemer G, et al. Digesting the crisis: autophagy and coronaviruses. Micro Cell. 2020;7:119–28.
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–83.
Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;10:1–4.
Mallapaty S. Why does the coronavirus spread so easily between people? Nature. 2020;579:183.
Douglas L, Reihill J, Ho MAJ, Martin S. Furin inhibition as a mechanism to reduce aberrant ENaC-mediated sodium transport and rehydrate the airways in cystic fibrosis lung disease. FASEB. 2019;33:802.26.
Ornatowski W, Poschet JF, Perkett E, Taylor-Cousar JL, Deretic V. Elevated furin levels in human cystic fibrosis cells result in hypersusceptibility to exotoxin A-induced cytotoxicity. J Clin Invest. 2007;117:3489–97.
Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 Inflammasome. Front Microbiol. 2019;10:50.
Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir Res. 2020;176:104742.
Ivanova T, Hardes K, Kallis S, Dahms SO, Than ME, Künzel S, et al. Optimization of substrate-analogue furin inhibitors. ChemMedChem. 2017;12:1953–68.
Anand P, Puranik A, Aravamudan M, Venkatakrishnan AJ, Soundararajan V. SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. Elife. 2020;9:e58603.
Poli P, Timpano S, Goffredo M, Padoan R, Badolato R. Asymptomatic case of Covid-19 in an infant with cystic fibrosis. J Cyst Fibros. 2020. https://doi.org/10.1016/j.jcf.2020.03.017.
Colombo C, Burgel PR, Gartner S, van Koningsbruggen-Rietschel S, Naehrlich L, Sermet-Gaudelus I, et al. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med. 2020;8:e35–e36.
Ecfs.eu. 2020. COVID-CF Project In Europe | European Cystic Fibrosis Society (ECFS). https://www.ecfs.eu/covid-cf-project-europe.
Cosgriff R, Ahern S, Bell SC, Brownlee K, Burgel PR, Byrnes C, et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. 2020. https://doi.org/10.1016/j.jcf.2020.04.012.
Bodas M, Vij N. Adapting proteostasis and autophagy for controlling the pathogenesis of cystic fibrosis lung disease. Front Pharm. 2019;10:20.
Poschet JF, Fazio JA, Timmins GS, Ornatowski W, Perkett E, Delgado M, et al. Endosomal hyperacidification in cystic fibrosis is due to defective nitric oxide-cylic GMP signalling cascade. EMBO Rep. 2006;7:553–9.
Sui H, Luo M, Miao Y, Cheng W, Wen S, Zhao B, et al. Cystic fibrosis transmembrane conductance regulator ameliorates lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/AKT/mTOR pathway in mice. Respir Physiol Neurobiol. 2020;273:103338.
Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–30.
Raju SV, Lin VY, Liu L, McNicholas CM, Karki S, Sloane PA, et al. The cystic fibrosis transmembrane conductance regulator potentiator ivacaftor augments mucociliary clearance abrogating cystic fibrosis transmembrane conductance regulator inhibition by cigarette smoke. Am J Respir Cell Mol Biol. 2017;56:99–108.
Çolak Y, Nordestgaard BG, Afzal S. Morbidity and mortality in carriers of the cystic fibrosis mutation. Eur Respir J. 2020. https://doi.org/10.1183/13993003.00558-2020.
Londino JD, Lazrak A, Noah JW, Aggarwal S, Bali V, Woodworth BA, et al. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. Faseb J. 2015;29:2712–25.
Londino JD, Lazrak A, Collawn JF, Bebok Z, Harrod KS, Matalon S. Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae. Am J Physiol Lung Cell Mol Physiol. 2017;313:L845–58.
Brand JD, Lazrak A, Trombley JE, Shei RJ, Adewale AT, Tipper JL, et al. Influenza-mediated reduction of lung epithelial ion channel activity leads to dysregulated pulmonary fluid homeostasis. JCI Insight. 2018;3:e123467.
Kiemer L, Lund O, Brunak S, Blom N. Coronavirus 3CLpro proteinase cleavage sites: possible relevance to SARS virus pathology. BMC Bioinforma. 2004;5:72.
Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DP has participated in international education and training programs supported by Vertex Pharmaceuticals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peckham, D., McDermott, M.F., Savic, S. et al. COVID-19 meets Cystic Fibrosis: for better or worse?. Genes Immun 21, 260–262 (2020). https://doi.org/10.1038/s41435-020-0103-y
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-020-0103-y
This article is cited by
-
Poor Respiratory Health Following Relapsing SARS-CoV-2 Infection in Children with Cystic Fibrosis: Correspondence
Indian Journal of Pediatrics (2022)
-
Poor Respiratory Health Following Relapsing SARS-CoV-2 Infection in Children with Cystic Fibrosis
Indian Journal of Pediatrics (2022)
-
Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic
BMC Pulmonary Medicine (2021)
-
Immune transcriptomes of highly exposed SARS-CoV-2 asymptomatic seropositive versus seronegative individuals from the Ischgl community
Scientific Reports (2021)
-
Cystic fibrosis improves COVID-19 survival and provides clues for treatment of SARS-CoV-2
Purinergic Signalling (2021)