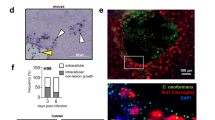
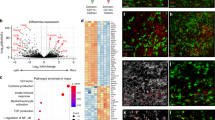
Abstract
Genetic mapping and genome-wide studies provide evidence for the association of several genetic polymorphisms with malaria, a complex pathological disease with multiple severity degrees. We have previously described Berr1and Berr2 as candidate genes identified in the WLA/Pas inbreed mouse strain predisposing to resistance to cerebral malaria (CM) induced by P. berghei ANKA. We report in this study the phenotypic and functional characteristics of a congenic strain we have derived for Berr2WLA allele on the C57BL/6JR (B6) background. B6.WLA-Berr2 was found highly resistant to CM compared to C57BL/6JR susceptible mice. The mechanisms associated with CM resistance were analyzed by combining genotype, transcriptomic and immune response studies. We found that B6.WLA-Berr2 mice showed a reduced parasite sequestration and blood–brain barrier disruption with low CXCR3+ T cell infiltration in the brain along with altered glial cell response upon P. berghei ANKA infection compared to B6. In addition, we have identified the CD300f, belonging to a family of Ig-like encoding genes, as a potential candidate associated with CM resistance. Microglia cells isolated from the brain of infected B6.WLA-Berr2 mice significantly expressed higher level of CD300f compared to CMS mice and were associated with inhibition of inflammatory response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Casanova J-L. Human genetic basis of interindividual variability in the course of infection. Proc Natl Acad Sci USA. 2015;112:E7118–27.
Casadevall A, Pirofski L. Microbiology: ditch the term pathogen. Nature. 2014;516:165–6.
Casanova J-L. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc Natl Acad Sci USA. 2015;112:E7128–37.
Casanova J-L, Abel L. The genetic theory of infectious diseases: a brief history and selected illustrations. Annu Rev Genom Hum Genet. 2013;14:215–43.
Hartl DL. The origin of malaria: mixed messages from genetic diversity. Nat Rev Microbiol. 2004;2:15–22.
Marquet S. Overview of human genetic susceptibility to malaria: From parasitemia control to severe disease. Infect Genet Evol. 2017;66:399–409.
WHO|World Malaria Report 2015. Geneva: World Health Organization; 2016.
Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigário AM. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect Immun. 2010;78:4033–9.
Grau GER, Craig AG. Cerebral malaria pathogenesis: revisiting parasite and host contributions. Future Microbiol. 2012;7:291–302.
Blanc A-L, Keswani T, Gorgette O, Bandeira A, Malissen B, Cazenave P-A, et al. Suppression of CD4+ effector responses by naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells contributes to experimental cerebral malaria. Infect Immun. 2016;84:329–38.
Shrivastava SK, Dalko E, Delcroix-Genete D, Herbert F, Cazenave P-A, Pied S. Uptake of parasite-derived vesicles by astrocytes and microglial phagocytosis of infected erythrocytes may drive neuroinflammation in cerebral malaria. Glia. 2017;65:75–92.
Dalko E, Genete D, Auger F, Dovergne C, Lambert C, Herbert F, et al. Heme dampens T-cell sequestration by modulating glial cell responses during rodent cerebral malaria. Brain Behav Immun. 2016;58:280–90.
Hill AV. The immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593–617.
Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–92.
Weatherall DJ. Genetic variation and susceptibility to infection: the red cell and malaria. Br J Haematol. 2008;141:276–86.
Rihet P, Abel L, Traoré Y, Traoré-Leroux T, Aucan C, Fumoux F. Human malaria: segregation analysis of blood infection levels in a suburban area and a rural area in Burkina Faso. Genet Epidemiol. 1998;15:435–50.
Migot-Nabias F, Mombo L, Luty A, Dubois B, Nabias R, Bisseye C, et al. Human genetic factors related to susceptibility to mild malaria in Gabon. Genes Immun. 2000;1:435–41.
Longley R, Smith C, Fortin A, Berghout J, McMorran B, Burgio G, et al. Host resistance to malaria: using mouse models to explore the host response. Mamm Genome. 2011;22:32–42.
de Oca MM, Engwerda C, Haque A. Plasmodium berghei ANKA (PbA) infection of C57BL/6J mice: a model of severe malaria. Methods Mol Biol. 2013;1031:203–13.
Bagot S, Campino S, Penha-Gonçalves C, Pied S, Cazenave P-A, Holmberg D. Identification of two cerebral malaria resistance loci using an inbred wild-derived mouse strain. Proc Natl Acad Sci USA. 2002;99:9919–23.
Campino S, Bagot S, Bergman M-L, Almeida P, Sepúlveda N, Pied S, et al. Genetic control of parasite clearance leads to resistance to Plasmodium berghei ANKA infection and confers immunity. Genes Immun. 2005;6:416–21.
Cadman ET, Abdallah AY, Voisine C, Sponaas A-M, Corran P, Lamb T, et al. Alterations of splenic architecture in malaria are induced independently of Toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect Immun. 2008;76:3924–31.
Shaw TN, Stewart-Hutchinson PJ, Strangward P, Dandamudi DB, Coles JA, Villegas-Mendez A, et al. Perivascular arrest of CD8+ T cells is a signature of experimental cerebral malaria. PLoS Pathog. 2015;11:e1005210.
Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81.
Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood. 2013;121:1951–60.
Clark GJ, Ju X, Azlan M, Tate C, Ding Y, Hart DNJ. The CD300 molecules regulate monocyte and dendritic cell functions. Immunobiology. 2009;214:730–6.
Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175:7989–95.
Rozenberg P, Reichman H, Moshkovits I, Munitz A. CD300 family receptors regulate eosinophil survival, chemotaxis, and effector functions. J Leukoc Biol. 2018;104:21–9.
Torre S, Langlais D, Gros P. Genetic analysis of cerebral malaria in the mouse model infected with Plasmodium berghei. Mamm Genome. 2018;29:488–506.
Nagayasu E, Nagakura K, Akaki M, Tamiya G, Makino S, Nakano Y, et al. Association of a determinant on mouse chromosome 18 with experimental severe Plasmodium berghei malaria. Infect Immun. 2002;70:512–6.
Caignard G, Eva MM, van Bruggen R, Eveleigh R, Bourque G, Malo D, et al. Mouse ENU mutagenesis to understand immunity to infection: methods, selected examples, and perspectives. Genes (Basel). 2014;5:887–925.
Foote SJ, Burt RA, Baldwin TM, Presente A, Roberts AW, Laural YL, et al. Mouse loci for malaria-induced mortality and the control of parasitaemia. Nat Genet. 1997;17:380–1.
Fortin A, Belouchi A, Tam MF, Cardon L, Skamene E, Stevenson MM, et al. Genetic control of blood parasitaemia in mouse malaria maps to chromosome 8. Nat Genet. 1997;17:382–3.
Ohno T, Ishih A, Kohara Y, Yonekawa H, Terada M, Nishimura M. Chromosomal mapping of the host resistance locus to rodent malaria (Plasmodium yoelii) infection in mice. Immunogenetics. 2001;53:736–40.
Fortin A, Stevenson MM, Gros P. Susceptibility to malaria as a complex trait: big pressure from a tiny creature. Hum Mol Genet. 2002;11:2469–78.
Min-Oo G, Willemetz A, Tam M, Canonne-Hergaux F, Stevenson MM, Gros P. Mapping of Char10, a novel malaria susceptibility locus on mouse chromosome 9. Genes Immun. 2009;11:113–23.
Bopp SER, Rodrigo E, González-Páez GE, Frazer M, Barnes SW, Valim C, et al. Identification of the Plasmodium berghei resistance locus 9 linked to survival on chromosome 9. Malar J. 2013;12:316.
Bongfen SE, Rodrigue-Gervais I-G, Berghout J, Torre S, Cingolani P, Wiltshire SA, et al. An N-ethyl-N-nitrosourea (ENU)-induced dominant negative mutation in the JAK3 kinase protects against cerebral malaria. PLoS ONE. 2012;7:e31012.
Ayodo G, Price AL, Keinan A, Ajwang A, Otieno MF, Orago ASS, et al. Combining evidence of natural selection with association analysis increases power to detect malaria-resistance variants. Am J Hum Genet. 2007;81:234–42.
Villegas-Mendez A, Greig R, Shaw TN, de Souza JB, Gwyer Findlay E, Stumhofer JS, et al. IFN-γ-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol. 2012;189:968–79.
Miu J, Mitchell AJ, Müller M, Carter SL, Manders PM, McQuillan JA, et al. Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. J Immunol. 2008;180:1217–30.
Nie CQ, Bernard NJ, Norman MU, Amante FH, Lundie RJ, Crabb BS, et al. IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog. 2009;5:e1000369.
Xie L, Choudhury GR, Winters A, Yang S-H, Jin K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur J Immunol. 2015;45:180–91.
Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192:871–80.
Herbert F, Tchitchek N, Bansal D, Jacques J, Pathak S, Bécavin C, et al. Evidence of IL-17, IP-10, and IL-10 involvement in multiple-organ dysfunction and IL-17 pathway in acute renal failure associated to Plasmodium falciparum malaria. J Transl Med. 2015;13:369.
Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1:749–58.
Belnoue E, Potter SM, Rosa DS, Mauduit M, Grüner AC, Kayibanda M, et al. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunol. 2008;30:544–53.
Lundie RJ, de Koning-Ward TF, Davey GM, Nie CQ, Hansen DS, Lau LS, et al. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells. Proc Natl Acad Sci USA. 2008;105:14509–14.
Claser C, De Souza JB, Thorburn SG, Grau GE, Riley EM, Rénia L, et al. Host resistance to plasmodium-induced acute immune pathology is regulated by interleukin-10 receptor signaling. Infect Immun. 2017;85:e00941.
Moshkovits I, Karo-Atar D, Itan M, Reichman H, Rozenberg P, Morgenstern-Ben-Baruch N, et al. CD300f associates with IL-4 receptor α and amplifies IL-4-induced immune cell responses. Proc Natl Acad Sci USA. 2015;112:8708–13.
Alvarez-Errico D, Aguilar H, Kitzig F, Brckalo T, Sayós J, López-Botet M. IREM-1 is a novel inhibitory receptor expressed by myeloid cells. Eur J Immunol. 2004;34:3690–701.
Besnard A-G, Guabiraba R, Niedbala W, Palomo J, Reverchon F, Shaw TN, et al. IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells. PLoS Pathog. 2015;11:e1004607.
Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404.
Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53:344–54.
Hirako IC, Ataide MA, Faustino L, Assis PA, Sorensen EW, Ueta H, et al. Splenic differentiation and emergence of CCR5+CXCL9+CXCL10+ monocyte-derived dendritic cells in the brain during cerebral malaria. Nat Commun. 2016;7:13277.
Pai S, Qin J, Cavanagh L, Mitchell A, El-Assaad F, Jain R, et al. Real-time imaging reveals the dynamics of leukocyte behaviour during experimental cerebral malaria pathogenesis. PLoS Pathog. 2014;10:e1004236.
Peluffo H, Solari-Saquieres P, Negro-Demontel ML, Francos-Quijorna I, Navarro X, López-Vales R, et al. CD300f immunoreceptor contributes to peripheral nerve regeneration by the modulation of macrophage inflammatory phenotype. J Neuroinflamm. 2015;12:145.
Peluffo H, Alí-Ruiz D, Ejarque-Ortíz A, Heras-Alvarez V, Comas-Casellas E, Martínez-Barriocanal A, et al. Overexpression of the immunoreceptor CD300f has a neuroprotective role in a model of acute brain injury. Brain Pathol. 2012;22:318–28.
Choi S-C, Simhadri VR, Tian L, Gil-Krzewska A, Krzewski K, Borrego F, et al. Cutting edge: mouse CD300f (CMRF-35-like molecule-1) recognizes outer membrane-exposed phosphatidylserine and can promote phagocytosis. J Immunol. 2011;187:3483–7.
Ejarque-Ortiz A, Solà C, Martínez-Barriocanal Á, Schwartz S, Martín M, Peluffo H, et al. The receptor CMRF35-like molecule-1 (CLM-1) enhances the production of LPS-induced pro-inflammatory mediators during microglial activation. PLoS ONE. 2015;10:e0123928.
Kim E-J, Lee S-M, Suk K, Lee W-H. CD300a and CD300f differentially regulate the MyD88 and TRIF-mediated TLR signalling pathways through activation of SHP-1 and/or SHP-2 in human monocytic cell lines. Immunology. 2012;135:226–35.
Lee S-M, Kim E-J, Suk K, Lee W-H. CD300F blocks both MyD88 and TRIF-mediated TLR signaling through activation of Src homology region 2 domain-containing phosphatase 1. J Immunol. 2011;186:6296–303.
Martínez-Barriocanal Á, Arcas-García A, Magallon-Lorenz M, Ejarque-Ortíz A, Negro-Demontel ML, Comas-Casellas E, et al. Effect of specific mutations in Cd300 complexes formation; potential implication of Cd300f in multiple sclerosis. Sci Rep. 2017;7:13544.
Nielsen R, Bustamante C, Clark AG, Glanowski S, Sackton TB, Hubisz MJ, et al. A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol. 2005;3:e170.
Biology of the laboratory mouse. Edited by George D. Snell. Dover Publications, Inc., New York, 1956 (Reprint of first edition, 1941). J Am Pharm Assoc. 1956;45:819.
Roland J, Soulard V, Sellier C, Drapier A-M, Di Santo JP, Cazenave P-A, et al. NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J Immunol. 2006;177:1229–39.
Acknowledgements
We gratefully acknowledge Dr. Sulabha Pathak for fruitful discussions, Dr. Xavier Montagutelli, Institut Pasteur Paris for the gift of WLA mice, and Oliver Gorgette for assistance in the generation of the recombinant B6xWLA mouse strains. Thanks are also due to Philippe Persoons and the staff of the animal facility of Institute Pasteur of Lille for maintenance and preservation of the mouse strains. We also thank Florent Auger and Nicolas Durieux from Platform of Biology and Health, CHRU Lille for MRI acquisition and analysis, Marine Swiathon for administrative support, and Shabdda Communication for scientific and language editing.
Funding
This work was supported by ARCir “Dynamique” Région Nord-Pas de Calais and the LabEx PARAFRAP: ANR-11-LABX-0024. TK is a recipient of PRESTIGE (Sanction No.2014-1-0043: Marie Curie Actions/FP7/PCOFUND-GA-2013-609102) and Région Haut-de-France (CNRS - Sanction No.147894) Post-doctoral Fellowship and Fondation des Treilles (Young Rresearcher Prize 2016)— “La Fondation des Treilles, créée par Anne Gruner Schlumberger, a notamment pour vocation d’ouvrir et de nourrir le dialogue entre les sciences et les arts afin de faire progresser la création et la recherche contemporaines. Elle accueille également des chercheurs et des écrivains dans le domaine des Treilles (Var) www.les-treilles.com”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: P-AC, JR, and SP; Investigation and design: JR, TK, DD-G, FH, LG, and HB-LT; Writing—original draft: PAC, TK, and SP; Writing—review & editing, Shabdda communication, TK, P-AC, JR, and SP; Funding acquisition and supervision, SP. All the authors discussed the results, commented on the article, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All animal work was conducted in strict accordance with the Directive 2010/63/EU of the European Parliament and Council “On the protection of animals used for scientific purposes.” All protocols were approved by the Ethical Committee of Institute Pasteur of Lille and the French Ministry of Agriculture (permit number A 75-15-28) and performed in compliance with the NIH Animal Welfare Assurance #A5476-01 issued on 02/07/2007. All efforts were taken to minimize animal suffering.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keswani, T., Roland, J., Herbert, F. et al. Expression of CD300lf by microglia contributes to resistance to cerebral malaria by impeding the neuroinflammation. Genes Immun 21, 45–62 (2020). https://doi.org/10.1038/s41435-019-0085-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-019-0085-9
This article is cited by
-
Management of cell death in parasitic infections
Seminars in Immunopathology (2021)