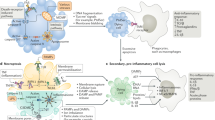
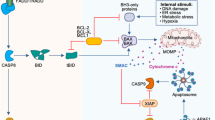
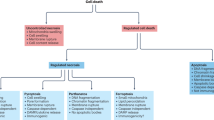
Abstract
The inhibitor of apoptosis proteins (IAPs) are best known for their ability to regulate cell survival and death processes. However, in addition to cell death, IAPs also act as innate immune sensors and modulate multiple pathways, such as autophagy and cell division. Many of these IAP functions are non-redundant even though they are based on the same molecular mechanism of action. These distinct functions of IAPs derive from their capacity to target specific substrates for ubiquitination and/or proteolytic cleavage. The unique functions of IAPs also derives from their unique cellular localizations, cell type and tissue-specific expression patterns. The diverse roles of IAPs are reflected by the fact that in humans the IAP family comprises eight distinct members. Genetic evidence from human pathologies also attests to the non-redundant functions of the IAPs since very diverse diseases arise upon aberrant IAP expression. In this review, we give an overview of the known mechanisms of action of the various IAPs, and focus on their specific roles in mediating innate immunity. We also look at the distinct phenotypes related to the dysregulation of the IAPs, and the human pathologies associated with each human IAP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:7.
Birnbaum MJ. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994;68:8.
Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2. ReviewS3009.
Galbán S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60.
Darding M, Meier P. IAPs: guardians of RIPK1. Cell Death Differ. 2012;19:58–66.
Damgaard RB, Nachbur U, Yabal M, Wong WW-L, Fiil BK, Kastirr M, et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol Cell. 2012;46:746–58.
Bertrand MJM, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700.
Gyrd-Hansen M, Darding M, Miasari M, Santoro MM, Zender L, Xue W, et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-κB as well as cell survival and oncogenesis. Nat Cell Biol. 2008;10:1309–17.
Blankenship JW, Varfolomeev E, Goncharov T, Fedorova AV, Kirkpatrick DS, Izrael-Tomasevic A, et al. Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2 1. Biochem J. 2009;417:149–65.
Annibaldi A, Wicky John S, Vanden Berghe T, Swatek KN, Ruan J, Liccardi G, et al. Ubiquitin-mediated regulation of RIPK1 kinase activity independent of IKK and MK2. Mol Cell. 2018;69:566–80.
Lopez J, John SW, Tenev T, Rautureau GJP, Hinds MG, Francalanci F, et al. CARD-mediated autoinhibition of cIAP1’s E3 ligase activity suppresses cell proliferation and migration. Mol Cell. 2011;42:569–83.
Chen Z, Naito M, Hori S, Mashima T, Yamori T, Tsuruo T. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochem Biophys Res Commun. 1999;264:847–54.
Hauser H-P, Bardroff M, Pyrowolakis G, Jentsch S. A giant ubiquitin-conjugating enzyme related to IAP apoptosis inhibitors. J Cell Biol. 1998;141:1415–22.
Leulier F, Lhocine N, Lemaitre B, Meier P. The drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol Cell Biol. 2006;26:7821–31.
Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, Ertürk-Hasdemir D, et al. Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for drosophila NF-κB signaling. Mol Cell. 2010;37:172–82.
Man SM, Kanneganti T-D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21.
Vance RE. The NAIP/NLRC4 inflammasomes. Curr Opin Immunol. 2015;32:84–9.
Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci. 2013;110:14408–13.
Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600.
Endrizzi MG. Genomic sequence analysis of the mouse naip gene array. Genome Res. 2000;10:1095–102.
Lalaoui N, Vaux DL. Recent advances in understanding inhibitor of apoptosis proteins. F1000Res. 2018;7:1889.
Estornes Y, Bertrand MJM. IAPs, regulators of innate immunity and inflammation. Semin Cell Dev Biol. 2015;39:106–14.
Wong W-L, Gentle I, Nachbur U, Anderton H, Vaux D, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-jB. Cell Death Differ. 2010;6:482–7.
Lawlor KE, Feltham R, Yabal M, Conos SA, Chen KW, Ziehe S, et al. XIAP loss triggers RIPK3- and caspase-8-driven IL-1β activation and cell death as a consequence of TLR-MyD88-Induced cIAP1-TRAF2 degradation. Cell Rep. 2017;20:668–82.
Yabal M, Müller N, Adler H, Knies N, Groß CJ, Damgaard RB, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–808.
Vince JE, Wong WW-L, Gentle I, Lawlor KE, Allam R, O’Reilly L, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–27.
Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–48.
Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block ripoptosome formation, a RIP1/Caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–63.
Zhang D-W, Shao J, Lin J, Zhang N, Lu B-J, Lin S-C, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6.
Wong WW-L, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–72.
Erlich Z, Shlomovitz I, Edry-Botzer L, Cohen H, Frank D, Wang H, et al. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat Immunol. 2019;20:397–406. https://doi.org/10.1038/s41590-019-0313-5.
Sun S-C. Non-canonical NF-κB signaling pathway. Cell Res. 2011;21:15.
Tegowski M, Baldwin A. Noncanonical NF-κB in cancer. Biomedicines. 2018;6:66.
Scott FL, Denault J-B, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645–55.
Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:5.
Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23:1627–35.
Deveraux QL. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–23.
Vucic D, Kaiser WJ, Miller LK. Inhibitor of apoptosis proteins physically interact with and block apoptosis induced by Drosophila proteins HID and GRIM. Mol Cell Biol. 1998;18:3300–9.
Herman MD, Moche M, Flodin S, Welin M, Trésaugues L, Johansson I, et al. Structures of BIR domains from human NAIP and cIAP2. Acta Crystallogr Sect F. 2009;65:1091–6.
Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–60.
Bauler LD, Duckett CS, O’Riordan MXD. XIAP regulates cytosol-specific innate immunity to listeria infection. PLoS Pathog. 2008;4:e1000142.
Fiil BK, Damgaard RB, Wagner SA, Keusekotten K, Fritsch M, Bekker-Jensen S, et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol Cell. 2013;50:818–30.
Hrdinka M, Fiil BK, Zucca M, Leske D, Bagola K, Yabal M, et al. CYLD limits Lys63- and Met1-linked ubiquitin at receptor complexes to regulate innate immune signaling. Cell Rep. 2016;14:2846–58.
Stafford CA, Lawlor KE, Heim VJ, Bankovacki A, Bernardini JP, Silke J, et al. IAPs regulate distinct innate immune pathways to co-ordinate the response to bacterial peptidoglycans. Cell Rep. 2018;22:1496–508.
Zumbrägel FK, Machtens DA, Curth U, Lüder CGK, Reubold TF, Eschenburg S. Survivin does not influence the anti-apoptotic action of XIAP on caspase-9. Biochem Biophys Res Commun. 2017;482:530–5.
Kitagawa M, Lee SH. The chromosomal passenger complex (CPC) as a key orchestrator of orderly mitotic exit and cytokinesis. Front Cell Dev Biol. 2015;3:14. https://doi.org/10.3389/fcell.2015.00014.
Fraser AG, James C, Evan GI, Hengartner MO. Caenorhabditis elegans inhibitor of apoptosis protein (IAP) homologue BIR-1 plays a conserved role in cytokinesis. Curr Biol. 1999;9:292–302.
Uren AG, Beilharz T, O’Connell MJ, Bugg SJ, van Driel R, Vaux DL, et al. Role for yeast inhibitor of apoptosis (IAP)-like proteins in cell division. Proc Natl Acad Sci. 1999;96:10170–5.
Martini E, Wittkopf N, Günther C, Leppkes M, Okada H, Watson AJ, et al. Loss of survivin in intestinal epithelial progenitor cells leads to mitotic catastrophe and breakdown of gut immune homeostasis. Cell Rep. 2016;14:1062–73.
Okada H, Bakal C, Shahinian A, Elia A, Wakeham A, Suh W-K, et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med. 2004;199:399–410.
Gravina G, Wasén C, Garcia-Bonete MJ, Turkkila M, Erlandsson MC, Töyrä Silfverswärd S, et al. Survivin in autoimmune diseases. Autoimmun Rev. 2017;16:845–55.
Hao Y, Sekine K, Kawabata A, Nakamura H, Ishioka T, Ohata H, et al. Apollon ubiquitinates SMAC and caspase-9 and has an essential cytoprotection function. Nat Cell Biol. 2004;6:849–60.
Ebner P, Poetsch I, Deszcz L, Hoffmann T, Zuber J, Ikeda F. The IAP family member BRUCE regulates autophagosome–lysosome fusion. Nat Commun. 2018;9:599. https://doi.org/10.1038/s41467-018-02823-x.
Vucic D, Deshayes K, Ackerly H, Pisabarro MT, Kadkhodayan S, Fairbrother WJ, et al. SMAC negatively regulates the anti-apoptotic activity of melanoma inhibitor of apoptosis (ML-IAP). J Biol Chem. 2002;277:12275–9.
Shin H, Renatus M, Eckelman BP, Nunes VA, Sampaio CAM, Salvesen GS. The BIR domain of IAP-like protein 2 is conformationally unstable: implications for caspase inhibition. Biochem J. 2005;385:1–10.
Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom. 2014;13:397–406.
Heng TSP, Painter MW, Elpek K, Lukacs-Kornek V, Mauermann N, Turley SJ, et al. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–4.
Burm SM, Zuiderwijk-Sick EA, ’t Jong AEJ, van der Putten C, Veth J, Kondova I, et al. Inflammasome-induced IL-1 secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J Neurosci. 2015;35:678–87.
Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci. 2013;110:2946–51.
Morón-Calvente V, Romero-Pinedo S, Toribio-Castelló S, Plaza-Díaz J, Abadía-Molina AC, Rojas-Barros DI, et al. Inhibitor of apoptosis proteins, NAIP, cIAP1 and cIAP2 expression during macrophage differentiation and M1/M2 polarization. PLoS ONE. 2018;13:e0193643.
Rijal D, Ariana A, Wight A, Kim K, Alturki NA, Aamir Z, et al. Differentiated macrophages acquire a pro-inflammatory and cell death–resistant phenotype due to increasing XIAP and p38-mediated inhibition of RipK1. J Biol Chem. 2018;293:11913–27.
Fukuda S, Foster RG, Porter SB, Pelus LM. The antiapoptosis protein survivin is associated with cell cycle entry of normal cord blood CD34ϩ cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood. 2002;100:10.
Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–74.
Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–24.
Rigaud S, Fondanèche M-C, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–4.
Marsh RA, Villanueva J, Kim M-O, Zhang K, Marmer D, Risma KA, et al. Patients with X-linked lymphoproliferative disease due to BIRC4 mutation have normal invariant natural killer T-cell populations. Clin Immunol. 2009;132:116–23.
Yang X, Kanegane H, Nishida N, Imamura T, Hamamoto K, Miyashita R, et al. Clinical and genetic characteristics of XIAP deficiency in Japan. J Clin Immunol. 2012;32:411–20.
Speckmann C, Lehmberg K, Albert MH, Damgaard RB, Fritsch M, Gyrd-Hansen M, et al. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol. 2013;149:133–41.
Speckmann C, Ehl S. XIAP deficiency is a mendelian cause of late-onset IBD. Gut. 2014;63:1031–2.
Zeissig Y, Petersen B-S, Milutinovic S, Bosse E, Mayr G, Peuker K, et al. XIAP variants in male Crohn’s disease. Gut. 2015;64:66–76.
Aguilar C, Latour S. X-linked inhibitor of apoptosis protein deficiency: more than an X-linked lymphoproliferative syndrome. J Clin Immunol. 2015;35:331–8.
Nielsen OH, LaCasse EC. How genetic testing can lead to targeted management of XIAP deficiency–related inflammatory bowel disease. Genet Med. 2017;19:133–43.
Latour S, Aguilar C. XIAP deficiency syndrome in humans. Semin Cell Dev Biol. 2015;39:115–23.
Damgaard RB, Fiil BK, Speckmann C, Yabal M, zur Stadt U, Bekker-Jensen S, et al. Disease-causing mutations in the XIAPBIR2 domain impair NOD2-dependent immune signalling: XLP2 mutations abrogate NOD2 signalling. EMBO Mol Med. 2013;5:1278–95.
Quaranta M, Wilson R, Serra EG, Pandey S, Schwerd T, Gilmour K et al. Genomics identifies XIAP deficiency in an adult patient with inflammatory bowel disease: The Clinical and Health Care Impact. Gastroenterology 2018. https://doi.org/10.1053/j.gastro.2018.03.069.
Ammann S, Elling R, Gyrd-Hansen M, Dückers G, Bredius R, Burns SO, et al. A new functional assay for the diagnosis of X-linked inhibitor of apoptosis (XIAP) deficiency: NOD2 stimulation diagnoses XIAP deficiency. Clin Exp Immunol. 2014;176:394–400.
Wada T, Kanegane H, Ohta K, Katoh F, Imamura T, Nakazawa Y, et al. Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency. Cytokine. 2014;65:74–78.
Christiansen M, Ammann S, Speckmann C, Mogensen TH. XIAP deficiency and MEFV variants resulting in an autoinflammatory lymphoproliferative syndrome. BMJ Case Rep. 2016;2016:bcr2016216922.
Gentle IE, Wong WW-L, Evans JM, Bankovacki A, Cook WD, Khan NR, et al. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-κB and activation of caspase-8. J Biol Chem. 2011;286:13282–91.
Bertrand MJM, Lippens S, Staes A, Gilbert B, Roelandt R, De Medts J, et al. cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor interacting proteins kinases 1 to 4 (RIP1–4). PLoS ONE. 2011;6:e22356.
Cuchet-Lourenço D, Eletto D, Wu C, Plagnol V, Papapietro O, Curtis J. et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science. 2018;361:810–3.
Li Y, Führer M, Bahrami E, Socha P, Klaudel-Dreszler M, Bouzidi A et al. Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc Natl Acad Sci. 2018;201813582.
Cantarini L, Lucherini OM, Muscari I, Frediani B, Galeazzi M, Brizi MG, et al. Tumour necrosis factor receptor-associated periodic syndrome (TRAPS): state of the art and future perspectives. Autoimmun Rev. 2012;12:38–43.
Lachmann HJ, Kuemmerle-Deschner JB, Hachulla E, Widmer A, Patel N. Use of Canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:10.
Gattorno M, Obici L, Cattalini M, Tormey V, Abrams K, Davis N, et al. Canakinumab treatment for patients with active recurrent or chronic TNF receptor-associated periodic syndrome (TRAPS): an open-label, phase II study. Ann Rheum Dis. 2017;76:173–8.
Kortmann J, Brubaker SW, Monack DM. Cutting edge: inflammasome activation in primary human macrophages is dependent on flagellin. J Immunol. 2015;195:815–9.
Duncan JA, Canna SW. The NLRC4 inflammasome. Immunol Rev. 2018;281:115–23.
Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–6.
Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med. 2014;211:2385–96.
Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, et al. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity. 2017;46:649–59.
Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46:1135–9.
Roy N, Mahadevan MS, McLean M, Shutter G, Yaraghi Z, Farahani R, et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80:167–78.
He J, Zhang Q-J, Lin Q-F, Chen Y-F, Lin X-Z, Lin M-T, et al. Molecular analysis of SMN1, SMN2, NAIP, GTF2H2, and H4F5 genes in 157 Chinese patients with spinal muscular atrophy. Gene. 2013;518:325–9.
Kano O, Tanaka K, Kanno T, Iwasaki Y, Ikeda J-E. Neuronal apoptosis inhibitory protein is implicated in amyotrophic lateral sclerosis symptoms. Sci Rep 2018. https://doi.org/10.1038/s41598-017-18627-w.
Kuo H-H, Ahmad R, Lee GQ, Gao C, Chen H-R, Ouyang Z, et al. Anti-apoptotic protein BIRC5 maintains survival of HIV-1-infected CD4+T cells. Immunity. 2018;48:1183.
Kim Y, Anderson JL, Lewin SR. Getting the “Kill” into “Shock and Kill”: strategies to eliminate latent HIV. Cell Host Microbe. 2018;23:14–26.
Pache L, Dutra MS, Spivak AM, Marlett JM, Murry JP, Hwang Y, et al. BIRC2/cIAP1 is a negative regulator of HIV-1 transcription and can be targeted by Smac mimetics to promote reversal of viral latency. Cell Host Microbe. 2015;18:345–53.
Campbell GR, Bruckman RS, Chu Y-L, Trout RN, Spector SA. SMAC mimetics induce autophagy-dependent apoptosis of HIV-1-infected resting memory CD4+T cells. Cell Host Microbe 2018. https://doi.org/10.1016/j.chom.2018.09.007.
Ebert G, Allison C, Preston S, Cooney J, Toe JG, Stutz MD, et al. Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proc Natl Acad Sci. 2015;112:5803–8.
Acknowledgements
We thank Marcello Turi for the graphical design of Fig. 1, and Prof. Percy Knolle for critical comments.
Funding
Matous Hrdinka is supported by The Czech Science Foundation (GA CR 18-24070Y), the Institutional Development Plan of University of Ostrava and The Ministry of Education, Youth and Sports (IRP03_2018‐2020), and MH CZ—DRO (FNOs/2017). Monica Yabal is supported by the German Research Foundation (DFG project number 405101514).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hrdinka, M., Yabal, M. Inhibitor of apoptosis proteins in human health and disease. Genes Immun 20, 641–650 (2019). https://doi.org/10.1038/s41435-019-0078-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-019-0078-8
This article is cited by
-
Molecular mechanisms of tumor resistance to radiotherapy
Molecular Cancer (2023)
-
cIAP1/TRAF2 interplay promotes tumor growth through the activation of STAT3
Oncogene (2023)
-
How does caspases regulation play role in cell decisions? apoptosis and beyond
Molecular and Cellular Biochemistry (2023)
-
Manifold role of ubiquitin in Helicobacter pylori infection and gastric cancer
Cellular and Molecular Life Sciences (2021)