Abstract
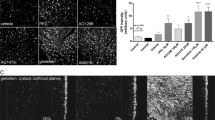
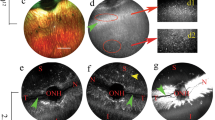
While many studies have investigated the use of recombinant adeno-associated vectors (rAAV) in the posterior chamber for treatment of inherited retinal diseases, fewer studies have looked at rAAV’s ability to transduce cells within the anterior chamber. This study focuses on evaluating the tropism and tolerability of three rAAV serotypes—rAAV2/6, rAAV2/9, and rAAV2/2[MAX]—expressing a green fluorescent protein (GFP) reporter following intracameral injection in the non-human primate (NHP) African green monkey (Chlorocebus sabaeus) model. Injection of high dose (1 × 1012 vg/eye) rAAV vector resulted in transient inflammation characterized by aqueous flare and cellular infiltrate that resolved without intervention in all serotypes. Post-mortem histology revealed widespread expression of GFP in cells of the trabecular meshwork and iris in high dose rAAV2/6, rAAV2/9, and particularly rAAV2/2[MAX] eyes, indicating that rAAV vectors of these serotypes have broad tropism for cells of the anterior chamber and may facilitate the treatment of blinding disorders, such as glaucoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data necessary to evaluate the conclusions within this paper are present in the paper and/or supplementary materials. Any other requests may be communicated to the corresponding author.
References
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8.
Liu J, Saghizadeh M, Tuli SS, Kramerov AA, Lewin AS, Bloom DC, et al. Different tropism of adenoviruses and adeno-associated viruses to corneal cells: implications for corneal gene therapy. Mol Vision. 2008;14:2087–96.
Morgan JT, Murphy CJ, Russell P. What do mechanotransduction, Hippo, Wnt, and TGFβ have in common? YAP and TAZ as key orchestrating molecules in ocular health and disease. Exp Eye Res. 2013;115:1–12.
Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. Academic Press; 2015;133:112–25.
Hao XD, Gao H, Xu WH, Shan C, Liu Y, Zhou ZX, et al. Systematically Displaying the Pathogenesis of Keratoconus via Multi-Level Related Gene Enrichment-Based Review. Front Med. 2022;8:770138.
Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, et al. Targeting Photoreceptors via Intravitreal Delivery Using Novel, Capsid-Mutated AAV Vectors. PLoS ONE. 2013;8:e62097.
Reid CA, Ertel KJ, Lipinski DM. Improvement of photoreceptor targeting via intravitreal delivery in mouse and human retina using combinatory rAAV2 capsid mutant vectors. Investig Ophthalmol Vis Sci. 2017;58:6429–39.
Bogner B, Boye SL, Min SH, Peterson JJ, Ruan Q, Zhang Z, et al. Capsid mutated adeno-associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS ONE. 2015;10:1–16.
Mohan RR, Schultz GS, Hong JW, Mohan RR, Wilson SE. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp Eye Res. 2003;76:373–83.
Hippert C, Ibanes S, Serratrice N, Court F, Malecaze F. Corneal Transduction by Intra-Stromal Injection of AAV Vectors In Vivo in the Mouse and Ex Vivo in Human Explants. PLoS ONE. 2012;7:35318.
Rodriguez-Estevez L, Asokan P, Borrás T. Transduction optimization of AAV vectors for human gene therapy of glaucoma and their reversed cell entry characteristics. Gene Ther. 2020;27:127–42.
Buie LKK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Investig Ophthalmol Vis Sci. 2010;51:236–48.
Lee SH, Sim KS, Kim CY, Park TK. Transduction Pattern of AAVs in the Trabecular Meshwork and Anterior-Segment Structures in a Rat Model of Ocular Hypertension. Mol Ther Methods Clin Dev. 2019;14:197–205.
Bogner B, Boye SL, Min SH, Peterson JJ, Ruan Q, Zhang Z, et al. Capsid mutated adeno-associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS ONE. 2015;10:e0128759.
Dore KM. Vervets in the Caribbean. The International Encyclopedia of Primatology: John Wiley & Sons, Inc.; 2017:1–3.
Goody R, Hu W, Whittaker S, Henry S, Brookes R, Struharik M, et al. Definition of Normal Ophthalmic Measures in the African Green Monkey. Investig Ophthalmol Visual Sci: ARVO Meeting Abstract. 2013;54:14–6382.
Reid CA, Lipinski DM. Small and Micro-Scale Recombinant Adeno-Associated Virus Production and Purification for Ocular Gene Therapy Applications. Methods Mol Biol. 2018;1715:19–31.
Zolotukhin S. Production of recombinant adeno-associated virus vectors. Hum Gene Ther. 2005;16:551–7.
Piedra J, Ontiveros M, Miravet S, Penalva C, Monfar M, Chillon M. Development of a Rapid, Robust, and Universal PicoGreen-Based Method to Titer Adeno-Associated Vectors. Hum Gene Ther Methods. 2015;26:35–42.
Corey TM, Woodley VV, O’connor M, Connolly E, Doyle S, Shrader S, et al. Evaluation of the Dose-Dependent Inflammatory Response and No-Observable Adverse Effect Level of Intravitreal Endotoxin in the African Green Monkey. Transl Vis Sci Technol. 2022;11:17.
Hussein N, Ostler B, Gormus BJ, Wolf R, Walsh GP. Intraocular pressure changes and postural changes of intraocular pressure in experimentally induced Hansen’s disease of Rhesus, Mangabey, and African green monkeys. Br J Ophthalmol. 1990;74:647–9.
Bouskila J, Palmour RM, Bouchard JF, Ptito M. Retinal structure and function in monkeys with fetal alcohol exposure. Exp Eye Res. 2018;177:55–64.
Bouquet C, Vignal Clermont C, Galy A, Fitoussi S, Blouin L, Munk MR, et al. Immune Response and Intraocular Inflammation in Patients with Leber Hereditary Optic Neuropathy Treated with Intravitreal Injection of Recombinant Adeno-Associated Virus 2 Carrying the ND4 Gene: A Secondary Analysis of a Phase 1/2 Clinical Trial. JAMA Ophthalmol. 2019;137:399–406.
Chan YK, Dick AD, Hall SM, Langmann T, Scribner CL, Mansfield BC. Inflammation in Viral Vector-Mediated Ocular Gene Therapy: A Review and Report From a Workshop Hosted by the Foundation Fighting Blindness. Trans Vis Sci Tech. 2021;10:3.
Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, et al. Cellular GFP Toxicity and Immunogenicity: Potential Confounders in in Vivo Cell Tracking Experiments. Stem Cell Rev. 2016;12:553.
McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–56.
Samaranch L, Sebastian WS, Kells AP, Salegio EA, Heller G, Bringas JR, et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther. 2014;22:329–37.
Ramsingh AI, Gray SJ, Reilly A, Koday M, Bratt D, Koday MT, et al. Sustained AAV9-mediated expression of a non-self protein in the CNS of non-human primates after immunomodulation. PLoS ONE. 2018;13:e0198154.
Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3:88ra54.
Gao G, Wang Q, Calcedo R, Mays L, Bells P, Wang L, et al. Adeno-Associated Virus-Mediated Gene Transferto Nonhuman Primate Liver Can Elicit Destructive Transgene-Specific T Cell Responses. Hum Gene Ther. 2009;20:930–42.
Boyd RF, Boye SL, Conlon TJ, Erger KE, Sledge DG, Langohr IM, et al. Reduced retinal transduction and enhanced transgene-directed immunogenicity with intravitreal delivery of rAAV following posterior vitrectomy in dogs. Gene Ther. 2016;23:548–56.
Bodh SA, Kumar V, Raina UK, Ghosh B, Thakar M. Inflammatory glaucoma. Oman J Ophthalmol. 2011;4:3–9.
Sen HN, Drye LT, Goldstein DA, Larson TA, Merrill PT, Pavan PR, et al. Hypotony in patients with uveitis: The Multicenter Uveitis Steroid Treatment (MUST) trial. Ocul Immunol Inflamm. 2012;20:104–12.
Tran VT, Mermoud A, Herbort CP. Appraisal and Management of Ocular Hypotony and Glaucoma Associated With Uveitis. Int Ophthalmol Clin. 2000;40:175–203.
Casanova MI, Young L, Park S, Kim S, Roszak K, Leonard BC, et al. Normal Corneal Thickness and Endothelial Cell Density in Rhesus Macaques (Macaca mulatta). Transl Vis Sci Technol. 2022;11:23.
Choi KE, Anh VTQ, Yun C, Kim YJ, Jung H, Eom H, et al. Normative data of ocular biometry, optical coherence tomography, and electrophysiology conducted for cynomolgus macaque monkeys. Transl Vis Sci Technol. 2021;10:14.
Denk Id N, Maloca Id P, Steiner G, Freichel C, Bassett S, Schnitzer TK, et al. Macular thickness measurements of healthy, naïve cynomolgus monkeys assessed with spectral-domain optical coherence tomography (SD-OCT). 2019;14:e0222850.
Chern KJ, Nettesheim ER, Reid CA, Li NW, Marcoe GJ, Lipinski DM. Prostaglandin-based rAAV-mediated glaucoma gene therapy in Brown Norway rats. Commun Biol. 2022;5:1169.
Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Franç Oise Montus M, et al. Prevalence of Serum IgG and Neutralizing Factors Against Adeno-Associated Virus (AAV) Types 1, 2, 5, 6, 8, and 9 in the Healthy Population: Implications for Gene Therapy Using AAV Vectors. Hum Gene Ther. 2010;21:661–789.
Acknowledgements
The authors would like to thank David Burke at Oregon Health & Science University for his assistance with the neutralizing antibody assays. We would also like to thank Gavin Marcoe for his assistance in vector manufacturing. In addition, the authors would like to thank the supporting staff at Virscio and the St. Kitts Biomedical Research Foundation.
Funding
This work was funded by intramural support from Medical College of Wisconsin’s Office of Research and NEI R01EY032478.
Author information
Authors and Affiliations
Contributions
Conceptualization: DML, MEO, MSL, KJC. Investigation: KJC, KZI, ZDG, MEO. Writing (Original Draft): KJC, DML, MEO. Writing (Revision and Editing): KJC, DML, MSL, MEO, KZI, ZDG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were approved by the Medical College of Wisconsin’s Institutional Animal Care and Use Committee and adhere to the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chern, K.J., Issac, K.Z., Gumbs, Z.D. et al. Tolerability and tropism of recombinant adeno-associated virus vectors in the African green monkey (Chlorocebus sabaeus) anterior chamber. Gene Ther 30, 714–722 (2023). https://doi.org/10.1038/s41434-023-00407-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-023-00407-z