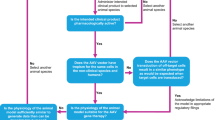
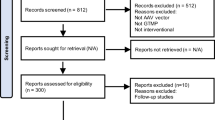
Abstract
Over 15 years after hepatotoxicity was first observed following administration of an adeno-associated virus (AAV) vector during a hemophilia B clinical trial, recent reports of treatment-associated neurotoxicity in animals and humans have brought the potential impact of AAV-associated toxicity back to prominence. In both pre-clinical studies and clinical trials, systemic AAV administration has been associated with neurotoxicity in peripheral nerve ganglia and spinal cord. Neurological signs have also been seen following direct AAV injection into the brain, both in non-human primates and in a clinical trial for late infantile Batten disease. Neurotoxic events appear variable across species, and preclinical animal studies do not fully predict clinical observations. Accumulating data suggest that AAV-associated neurotoxicity may be underdiagnosed and may differ between species in terms of frequency and/or severity. In this review, we discuss the different animal models that have been used to demonstrate AAV-associated neurotoxicity, its potential causes and consequences, and potential approaches to blunt AAV-associated neurotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
No datasets were generated for this review. All works reviewed in this study are referenced in the published article.
References
Colon-Thillet R, Jerome KR, Stone D. Optimization of AAV vectors to target persistent viral reservoirs. Virol J. 2021;18:85.
Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–78.
Hudry E, Vandenberghe LH. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;101:839–62.
Morris JA, Boshoff CH, Schor NF, Wong LM, Gao G, Davidson BL. Next-generation strategies for gene-targeted therapies of central nervous system disorders: a workshop summary. Mol Ther. 2021;29:3332–44.
Schuster DJ, Dykstra JA, Riedl MS, Kitto KF, Belur LR, McIvor RS, et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat. 2014;8:42.
Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29:285–98.
Hordeaux J, Buza EL, Dyer C, Goode T, Mitchell TW, Richman L, et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum Gene Ther. 2020;31:808–18.
Hordeaux J, Buza EL, Jeffrey B, Song C, Jahan T, Yuan Y, et al. MicroRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci Transl Med. 2020;12:eaba9188.
Hordeaux J, Hinderer C, Buza EL, Louboutin JP, Jahan T, Bell P, et al. Safe and sustained expression of human iduronidase after intrathecal administration of adeno-associated virus serotype 9 in infant Rhesus monkeys. Hum Gene Ther. 2019;30:957–66.
Hordeaux J, Hinderer C, Goode T, Buza EL, Bell P, Calcedo R, et al. Toxicology study of intra-cisterna magna adeno-associated virus 9 expressing iduronate-2-sulfatase in Rhesus macaques. Mol Ther Methods Clin Dev. 2018;10:68–78.
Hordeaux J, Hinderer C, Goode T, Katz N, Buza EL, Bell P, et al. Toxicology study of intra-cisterna magna adeno-associated virus 9 expressing human alpha-L-iduronidase in Rhesus macaques. Mol Ther Methods Clin Dev. 2018;10:79–88.
Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol Ther. 2018;26:664–8.
Bolt MW, Brady JT, Whiteley LO, Khan KN. Development challenges associated with rAAV-based gene therapies. J Toxicol Sci. 2021;46:57–68.
EMA. Zolgensma assessment report. https://www.ema.europa.eu/en/documents/assessment-report/zolgensma-epar-public-assessment-report_en.pdf, 2020.
Palazzi X, Pardo ID, Sirivelu MP, Newman L, Kumpf SW, Qian J, et al. Biodistribution and tolerability of AAV-PHP.B-CBh-SMN1 in wistar han rats and cynomolgus macaques reveal different toxicologic profiles. Hum Gene Ther. 2022;33:175–87.
Hinderer C, Bell P, Louboutin JP, Katz N, Zhu Y, Lin G, et al. Neonatal tolerance induction enables accurate evaluation of gene therapy for MPS I in a canine model. Mol Genet Metab. 2016;119:124–30.
Marco S, Haurigot V, Jaen ML, Ribera A, Sanchez V, Molas M, et al. Seven-year follow-up of durability and safety of AAV CNS gene therapy for a lysosomal storage disorder in a large animal. Mol Ther Methods Clin Dev. 2021;23:370–89.
Hordeaux J, Jeffrey BA, Jian J, Choudhury GR, Michalson K, Mitchell TW, et al. Efficacy and safety of a krabbe disease gene therapy. Hum Gene Ther. 2022.
Buss N, Lanigan L, Zeller J, Cissell D, Metea M, Adams E, et al. Characterization of AAV-mediated dorsal root ganglionopathy. Mol Ther Methods Clin Dev. 2022;24:342–54.
Fader KA, Pardo ID, Kovi RC, Somps CJ, Wang HH, Vaidya VS, et al. Circulating neurofilament light chain as a promising biomarker of AAV-induced dorsal root ganglia toxicity in nonclinical toxicology species. Mol Ther Methods Clin Dev. 2022;25:264–77.
Johnston S, Parylak SL, Kim S, Mac N, Lim C, Gallina I, et al. AAV ablates neurogenesis in the adult murine hippocampus. Elife. 2021;10:e59291.
Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS, et al. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol Ther. 2013;21:158–66.
Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13:517–27.
Samaranch L, Sebastian WS, Kells AP, Salegio EA, Heller G, Bringas JR, et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther. 2014;22:329–37.
Zerah M, Piguet F, Colle MA, Raoul S, Deschamps JY, Deniaud J, et al. Intracerebral gene therapy using AAVrh.10-hARSA recombinant vector to treat patients with early-onset forms of metachromatic leukodystrophy: preclinical feasibility and safety assessments in nonhuman primates. Hum Gene Ther Clin Dev. 2015;26:113–24.
Crystal R. Clinical and nonclinical consequences of direct CNS parenchymal administration of AAV vectors: https://www.fda.gov/media/151998/download, 2021.
Rosenberg JB, Chen A, De BP, Dyke JP, Ballon DJ, Monette S, et al. Safety of direct intraparenchymal AAVrh.10-mediated central nervous system gene therapy for metachromatic leukodystrophy. Hum Gene Ther. 2021;32:563–80.
Golebiowski D, van der Bom IMJ, Kwon CS, Miller AD, Petrosky K, Bradbury AM, et al. Direct intracranial injection of AAVrh8 encoding monkey beta-N-acetylhexosaminidase causes neurotoxicity in the primate brain. Hum Gene Ther. 2017;28:510–22.
Keiser MS, Ranum PT, Yrigollen CM, Carrell EM, Smith GR, Muehlmatt AL, et al. Toxicity after AAV delivery of RNAi expression constructs into nonhuman primate brain. Nat Med. 2021;27:1982–9.
Davidson MK, Lindsey JR, Davis JK. Requirements and selection of an animal model. Isr J Med Sci. 1987;23:551–5.
Eaton SL, Wishart TM. Bridging the gap: large animal models in neurodegenerative research. Mamm Genome. 2017;28:324–37.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–7.
Kuzmin DA, Shutova MV, Johnston NR, Smith OP, Fedorin VV, Kukushkin YS, et al. The clinical landscape for AAV gene therapies. Nat Rev Drug Discov. 2021;20:173–4.
Chand DH, Zaidman C, Arya K, Millner R, Farrar MA, Mackie FE, et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J Pediatr. 2021;231:265–8.
Feldman AG, Parsons JA, Dutmer CM, Veerapandiyan A, Hafberg E, Maloney N, et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J Pediatr. 2020;225:252–8 e1.
High-dose AAV gene therapy deaths. Nat Biotechnol. 2020; 38:910. https://doi.org/10.1038/s41587-020-0642-9
Morales L, Gambhir Y, Bennett J, Stedman HH. Broader implications of progressive liver dysfunction and lethal sepsis in two boys following systemic high-dose AAV. Mol Ther. 2020;28:1753–5.
Astellas. Astellas reports update to September 1 announcement on the ASPIRO clinical trial of AT132 in patients with X-linked myotubular myopathy. https://newsroom.astellas.us/2021-09-14-Astellas-Reports-Update-to-September-1-Announcement-on-the-ASPIRO-Clinical-Trial-of-AT132-in-Patients-with-X-linked-Myotubular-Myopathy.
Bonnemann C. AAV Related immunological safety and toxicity: preliminary clinical observations in the GAN and MTM1 trials. In Proceedings of virtual workshop on systemic immunogenicity considerations of AAV-mediated gene therapy, NIH, NCATS: https://videocast.nih.gov/watch=3854, 2020.
Mueller C, Berry JD, McKenna-Yasek DM, Gernoux G, Owegi MA, Pothier LM, et al. SOD1 suppression with adeno-associated virus and MicroRNA in familial ALS. N Engl J Med. 2020;383:151–8.
Mullard A. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat Rev Drug Discov. 2021;20:804–5.
Sondhi D, Kaminsky SM, Hackett NR, Pagovich OE, Rosenberg JB, De BP, et al. Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2. Sci Transl Med. 2020;12:eabb5413.
Reinhold AK, Rittner HL. Barrier function in the peripheral and central nervous system-a review. Pflugers Arch. 2017;469:123–34.
Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol. 2011;686:149–73.
Montague-Cardoso K, Malcangio M. Changes in blood-spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain. Pain Rep. 2021;6:e879.
Liu D, Zhu M, Zhang Y, Diao Y. Crossing the blood-brain barrier with AAV vectors. Metab Brain Dis. 2021;36:45–52.
Penaud-Budloo M, Francois A, Clement N, Ayuso E. Pharmacology of recombinant adeno-associated virus production. Mol Ther Methods Clin Dev. 2018;8:166–80.
Rumachik NG, Malaker SA, Poweleit N, Maynard LH, Adams CM, Leib RD, et al. Methods matter: standard production platforms for recombinant AAV produce chemically and functionally distinct vectors. Mol Ther Methods Clin Dev. 2020;18:98–118.
Robert MA, Chahal PS, Audy A, Kamen A, Gilbert R, Gaillet B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol J. 2017;12:1600193.
Kondratova L, Kondratov O, Ragheb R, Zolotukhin S. Removal of endotoxin from rAAV samples using a simple detergent-based protocol. Mol Ther Methods Clin Dev. 2019;15:112–9.
Schnodt M, Buning H. Improving the quality of adeno-associated viral vector preparations: the challenge of product-related impurities. Hum Gene Ther Methods. 2017;28:101–8.
Wright JF. Codon modification and PAMPs in clinical AAV vectors: the tortoise or the hare? Mol Ther. 2020;28:701–3.
Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19:1971–80.
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65.
Pattali R, Mou Y, Li XJ. AAV9 Vector: a Novel modality in gene therapy for spinal muscular atrophy. Gene Ther. 2019;26:287–95.
Lykken EA, Shyng C, Edwards RJ, Rozenberg A, Gray SJ. Recent progress and considerations for AAV gene therapies targeting the central nervous system. J Neurodev Disord. 2018;10:16.
Adachi K, Enoki T, Kawano Y, Veraz M, Nakai H. Drawing a high-resolution functional map of adeno-associated virus capsid by massively parallel sequencing. Nat Commun. 2014;5:3075.
Earley LF, Conatser LM, Lue VM, Dobbins AL, Li C, Hirsch ML, et al. Adeno-associated virus serotype-specific inverted terminal repeat sequence role in vector transgene expression. Hum Gene Ther. 2020;31:151–62.
Pan X, Yue Y, Boftsi M, Wasala LP, Tran NT, Zhang K, et al. Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther. 2022;29:333–45.
Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, et al. Cellular GFP toxicity and immunogenicity: potential confounders in in vivo cell tracking experiments. Stem Cell Rev Rep. 2016;12:553–9.
Hagedorn C, Schnodt-Fuchs M, Boehme P, Abdelrazik H, Lipps HJ, Buning H. S/MAR element facilitates episomal long-term persistence of adeno-associated virus vector genomes in proliferating cells. Hum Gene Ther. 2017;28:1169–79.
Powell SK, Rivera-Soto R, Gray SJ. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov Med. 2015;19:49–57.
Sun X, Yu X, Zhang L, Zhao W, Wang M, Zhang Y, et al. Comparison of the expression and toxicity of AAV2/9 carrying the human A53T alpha-synuclein gene in presence or absence of WPRE. Heliyon. 2021;7:e06302.
Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol. 2002;76:11605–11.
Ertl HCJ. T cell-mediated immune responses to AAV and AAV vectors. Front Immunol. 2021;12:666666.
Herzog RW. Complexity of immune responses to AAV transgene products—example of factor IX. Cell Immunol. 2019;342:103658.
Muhuri M, Maeda Y, Ma H, Ram S, Fitzgerald KA, Tai PW, et al. Overcoming innate immune barriers that impede AAV gene therapy vectors. J Clin Investig. 2021;131:e143780.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–65.
Perez BA, Shutterly A, Chan YK, Byrne BJ, Corti M. Management of neuroinflammatory responses to AAV-mediated gene therapies for neurodegenerative diseases. Brain Sci. 2020;10:119.
Suriano CM, Verpeut JL, Kumar N, Ma J, Jung C, Boulanger LM. Adeno-associated virus (AAV) reduces cortical dendritic complexity in a TLR9-dependent manner. bioRxiv.org 2021. https://doi.org/10.1101/2021.09.28.462148.
Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–68.
Faust SM, Bell P, Cutler BJ, Ashley SN, Zhu Y, Rabinowitz JE, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Investig. 2013;123:2994–3001.
Xiang Z, Kurupati RK, Li Y, Kuranda K, Zhou X, Mingozzi F, et al. The Effect of CpG sequences on capsid-specific CD8(+) T cell responses to AAV vector gene transfer. Mol Ther. 2020;28:771–83.
Bertolini TB, Shirley JL, Zolotukhin I, Li X, Kaisho T, Xiao W, et al. Effect of CpG depletion of vector genome on CD8(+) T cell responses in AAV gene therapy. Front Immunol. 2021;12:672449.
Chan YK, Wang SK, Chu CJ, Copland DA, Letizia AJ, Costa Verdera H, et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci Transl Med. 2021;13:eabd3438.
Konkle BA, Walsh CE, Escobar MA, Josephson NC, Young G, von Drygalski A, et al. BAX 335 hemophilia B gene therapy clinical trial results: potential impact of CpG sequences on gene expression. Blood. 2021;137:763–74.
Ghemrawi R, Khair M. Endoplasmic reticulum stress and unfolded protein response in neurodegenerative diseases. Int J Mol Sci. 2020;21:6127.
Prasad V, Greber UF. The endoplasmic reticulum unfolded protein response—homeostasis, cell death and evolution in virus infections. FEMS Microbiol Rev. 2021;45:fuab016.
Zolotukhin I, Markusic DM, Palaschak B, Hoffman BE, Srikanthan MA, Herzog RW. Potential for cellular stress response to hepatic factor VIII expression from AAV vector. Mol Ther Methods Clin Dev. 2016;3:16063.
Balakrishnan B, Sen D, Hareendran S, Roshini V, David S, Srivastava A, et al. Activation of the cellular unfolded protein response by recombinant adeno-associated virus vectors. PLoS One. 2013;8:e53845.
Buning H, Srivastava A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol Ther Methods Clin Dev. 2019;12:248–65.
Domenger C, Grimm D. Next-generation AAV vectors-do not judge a virus (only) by its cover. Hum Mol Genet. 2019;28:R3–14.
Monteys AM, Hundley AA, Ranum PT, Tecedor L, Muehlmatt A, Lim E, et al. Regulated control of gene therapies by drug-induced splicing. Nature. 2021;596:291–5.
Geisler A, Fechner H. MicroRNA-regulated viral vectors for gene therapy. World J Exp Med. 2016;6:37–54.
Sinnett SE, Boyle E, Lyons C, Gray SJ. Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice. Brain. 2021;144:3005–19.
Watson ZL, Ertel MK, Lewin AS, Tuli SS, Schultz GS, Neumann DM, et al. Adeno-associated virus vectors efficiently transduce mouse and rabbit sensory neurons coinfected with herpes simplex virus 1 following peripheral inoculation. J Virol. 2016;90:7894–901.
Fooden J. Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780). In: Fieldiana, vol. 96. Field Museum of Natural History, 2000, pp 1-180.
Amato R, Gardin JF, Tooze JA, Cline JM. Organ weights in relation to age and sex in Cynomolgus Monkeys (Macaca fascicularis). Toxicol Pathol. 2022;50:574–90.
Herndon JG, Tigges J, Klumpp SA, Anderson DC. Brain weight does not decrease with age in adult rhesus monkeys. Neurobiol Aging. 1998;19:267–72.
Dearing J, Conte A, Brooks C, AZImina A, Rivas R, Brich SM, et al. Characterization of enterovirus D68 infection in four nonhuman primate species. bioRxiv.org 2022. https://doi.org/10.1101/2022.04.16.487524
Acknowledgements
We thank the members of the Jerome lab for thoughtful discussions.
Funding
Research in our laboratory is funded by NIH grants R21AI117519, R01AI132599, UM1 AI126623, amfAR, the Caladan Foundation, over 1600 individual donors, and, in part, by a developmental grant from the University of Washington Center for AIDS Research (CFAR), an NIH-funded program under award number P30 AI 027757, which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK), and in part by NIH/NCI Cancer Center Support Grant P30 CA015704.
Author information
Authors and Affiliations
Contributions
DS, MA, and KRJ conceived and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
KRJ is a paid advisor and holds equity in Excision Biosciences, has sponsored research agreements with Excision Biosciences and Emendo Biotherapeutics, and is co-inventor of patents held by Fred Hutch regarding AAV-delivered gene therapy. DS and MA have sponsored research agreements with Excision Biosciences.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stone, D., Aubert, M. & Jerome, K.R. Adeno-associated virus vectors and neurotoxicity—lessons from preclinical and human studies. Gene Ther (2023). https://doi.org/10.1038/s41434-023-00405-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41434-023-00405-1