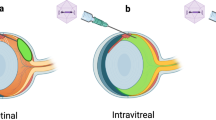
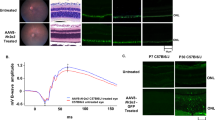
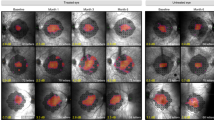
Abstract
In humans, mutations in the beta subunit of cGMP-phosphodiesterase type 6 (PDE6B) cause autosomal recessive retinitis pigmentosa (RP), which typically has an aggressive clinical course of early-onset severe vision loss due to rapid photoreceptor degeneration. In this study, we describe the generation of a novel Pde6b-deficient rat model using CRISPR-Cas9 genome editing. We characterize the model at multiple time points using clinical imaging modalities as well as histology with immunohistochemistry to show rapid photoreceptor degeneration compared to wild-type and heterozygous animals. We describe the manufacture of two different adeno-associated viral (AAV) vectors (AAV2/1, AAV2/5) under current Good Manufacturing Practices (cGMP) and demonstrate their ability to drive human PDE6B expression in vivo. We further demonstrate the ability of AAV-mediated subretinal gene therapy to delay photoreceptor loss in Pde6b-deficient rats compared to untreated controls. However, severe progressive photoreceptor loss was noted even in treated eyes, likely due to the aggressive nature of the disease. These data provide useful preclinical data to guide the development of potential human gene therapy for PDE6B-associated RP. In addition, the rapid photoreceptor degeneration of the Pde6b-deficient rat with intact inner retina may provide a useful model for the study of cell replacement strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All pertinent data generated and analyzed during this study can be found within the published manuscript and supplementary data file.
References
McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4:130–4.
Stone EM, Andorf JL, Whitmore SS, DeLuca AP, Giacalone JC, Streb LM, et al. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology. 2017;124:1314–31.
McLaughlin ME, Ehrhart TL, Berson EL, Dryja TP. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA. 1995;92:3249–53.
Kuehlewein L, Zobor D, Stingl K, Kempf M, Nasser F, Bernd A, et al. Clinical phenotype of PDE6B-associated retinitis pigmentosa. Int J Mol Sci. 2021;22:2374.
Kim YN, Song JS, Oh SH, Kim YJ, Yoon YH, Seo EJ, et al. Clinical characteristics and disease progression of retinitis pigmentosa associated with PDE6B mutations in Korean patients. Sci Rep. 2020;10:19540.
Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–80.
Nishiguchi KM, Carvalho LS, Rizzi M, Powell K, Holthaus SM, Azam SA, et al. Gene therapy restores vision in rd1 mice after removal of a confounding mutation in Gpr179. Nat Commun. 2015;6:6006.
Tucker BA, Burnight ER, Cranston CM, Ulferts MJ, Luse MA, Westfall T, et al. Development and biological characterization of a clinical gene transfer vector for the treatment of MAK-associated retinitis pigmentosa. Gene Ther. 2022;29:259–88.
Boswell CA, Mundo EE, Ulufatu S, Bumbaca D, Cahaya HS, Majidy N, et al. Comparative physiology of mice and rats: radiometric measurement of vascular parameters in rodent tissues. Mol Pharm. 2014;11:1591–8.
Yang JM, Chung S, Yun K, Kim B, So S, Kang S, et al. Long-term effects of human induced pluripotent stem cell-derived retinal cell transplantation in Pde6b knockout rats. Exp Mol Med. 2021;53:631–42.
Han IC, Burnight ER, Ulferts MJ, Worthington KS, Russell SR, Sohn EH, et al. Helper-dependent adenovirus transduces the human and rat retina but elicits an inflammatory reaction when delivered subretinally in rats. Hum Gene Ther. 2019;30:1371–84.
Han IC, Cheng JL, Burnight ER, Ralston CL, Fick JL, Thomsen GJ, et al. Retinal tropism and transduction of adeno-associated virus varies by serotype and route of delivery (intravitreal, subretinal, or suprachoroidal) in rats. Hum Gene Ther. 2020;31:1288–99.
Wiley LA, Burnight ER, Drack AV, Banach BB, Ochoa D, Cranston CM, et al. Using patient-specific induced pluripotent stem cells and wild-type mice to develop a gene augmentation-based strategy to treat CLN3-associated retinal degeneration. Hum Gene Ther. 2016;27:835–46.
Han IC, Burnight ER, Kaalberg EE, Boyce TM, Stone EM, Fingert JH, et al. Chimeric helper-dependent adenoviruses transduce retinal ganglion cells and muller cells in human retinal explants. J Ocul Pharmacol Ther. 2021;37:575–9.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Yeo JH, Jung BK, Lee H, Baek IJ, Sung YH, Shin HS, et al. Development of a Pde6b gene knockout rat model for studies of degenerative retinal diseases. Invest Ophthalmol Vis Sci. 2019;60:1519–26.
Wiley LA, Burnight ER, Kaalberg EE, Jiao C, Riker MJ, Halder JA, et al. Assessment of adeno-associated virus serotype tropism in human retinal explants. Hum Gene Ther. 2018;29:424–36.
Jomary C, Vincent KA, Grist J, Neal MJ, Jones SE. Rescue of photoreceptor function by AAV-mediated gene transfer in a mouse model of inherited retinal degeneration. Gene Ther. 1997;4:683–90.
Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, et al. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–54.
Thompson JR, Worthington KS, Green BJ, Mullin NK, Jiao C, Kaalberg EE, et al. Two-photon polymerized poly(caprolactone) retinal cell delivery scaffolds and their systemic and retinal biocompatibility. Acta Biomater. 2019;94:204–18.
Giacalone JC, Wiley LA, Burnight ER, Songstad AE, Mullins RF, Stone EM, et al. Concise review: patient-specific stem cells to interrogate inherited eye disease. Stem Cells Transl Med. 2016;5:132–40.
Wiley LA, Burnight ER, Songstad AE, Drack AV, Mullins RF, Stone EM, et al. Patient-specific induced pluripotent stem cells (iPSCs) for the study and treatment of retinal degenerative diseases. Prog Retin Eye Res. 2015;44:15–35.
Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, et al. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci Rep. 2016;6:30742.
Funding
University of Iowa Institute for Vision Research, University of Iowa, Iowa City, Iowa; Research to Prevent Blindness, New York City, New York; Carver cGMP Operational Endowment for Gene Therapy, University of Iowa, Iowa City, Iowa; National Institutes of Health Grant (NIH; Bethesda, MD, USA): P30 EY025580.
Author information
Authors and Affiliations
Contributions
ICH and LAW conceived of, designed, performed experiments, analyzed data and wrote the manuscript. ICH, LAW, DO, MJL, BEH and KMS acquired samples and helped perform experiments. EMS and RFM provided funding support, edited and approved the final manuscript. BAT conceived the study, provided funding support, as well as edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
As also stated in the methods section, all rat experiments were conducted with the approval of the University of Iowa Animal Care and Use Committee (Animal welfare assurance #1031317) and were consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, I.C., Wiley, L.A., Ochoa, D. et al. Characterization of a novel Pde6b-deficient rat model of retinal degeneration and treatment with adeno-associated virus (AAV) gene therapy. Gene Ther 30, 362–368 (2023). https://doi.org/10.1038/s41434-022-00365-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-022-00365-y
This article is cited by
-
Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina
Nature Communications (2023)