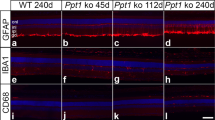
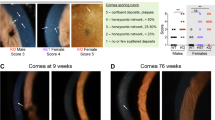
Abstract
Farber disease (FD) is a rare monogenic lysosomal storage disorder caused by mutations in ASAH1 that results in a deficiency of acid ceramidase (ACDase) activity and the abnormal systemic accumulation of ceramide species, leading to multi-system organ failure involving neurological decline and retinopathy. Here we describe the effects of rAAV-mediated ASAH1 over-expression on the progression of retinopathy in a mouse model of FD (Asah1P361R/P361R) and its littermate controls (Asah1+/+ and Asah1+/P361R). Using a combination of non-invasive multimodal imaging, electrophysiology, post-mortem histology and mass spectrometry we demonstrate that ASAH1 over-expression significantly reduces central retinal thickening, ceramide accumulation, macrophage activation and limits fundus hyper-reflectivity and auto-fluorescence in FD mice, indicating rAAV-mediated over-expression of biologically active ACDase protein is able to rescue the anatomical retinal phenotype of Farber disease. Unexpectedly, ACDase over-expression in Asah1+/+ and Asah1+/P361R control eyes was observed to induce abnormal fundus hyper-reflectivity, auto-fluorescence and retinal thickening that closely resembles a FD phenotype. This study represents the first evidence of a gene therapy for Farber disease-related retinopathy. Importantly, the described gene therapy approach could be used to preserve vision in FD patients synergistically with broader enzyme replacement strategies aimed at preserving life.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
References
Yu FPS, Amintas S, Levade T, Medin JA. Acid ceramidase deficiency: Farber disease and SMA-PME. Orphanet J Rare Dis. 2018;13:1–19.
Sugita M, Dulaney JT, Moser HW. Ceramidase deficiency in Farber’s disease (Lipogranulomatosis). Science. 1972;178:1100–2.
Koch J, Gärtner S, Li C, Quintern LE, Bernardo K, Levran O. et al. Molecular cloning and characterization of a full-length complementary DNAencoding human acid ceramidase: Identification of the first molecular lesion causing farber disease. J Biol Chem. 1996;271:33110–5.
Sands MS. Farber disease: Understanding a fatal childhood disorder and dissecting ceramide biology. EMBO Mol. Med. 2013;5:799–801.
Zielonka M, Garbade SF, Kölker S, Hoffmann GF, Ries M. A cross-sectional quantitative analysis of the natural history of Farber disease: An ultra-orphan condition with rheumatologic and neurological cardinal disease features. Genet. Med. 2018;20:524–30.
Zetterström R. Disseminated Lipogranulomatosis (Farber’s Disease). Acta Paediatr. 1958;47:501–10.
Tanaka T, Takahashi K, Hakozaki H, Kimoto H, Suzuki Y. Farber’s Disease (Disseminated Lipogranulomatosis) - A Pathological, Histochemical and Ultrastructural Study-. Pathol. Int. 1979;29:135–55.
Zarbin MA, Green WR, Moser AB, Tiffany C. Increased Levels of Ceramide in the Retina of a Patient With Farber’s Disease. Arch. Ophthalmol. 1988;106:1163–1163.
Alamri AS, Alshowaeir DA, AlFaiz AA, Mousawi AI, Mahmoud FH, Alhashim AA. et al. Optic Nerve Involvement in Farber Lipogranulomatosis: Expanding the Phenotypic Spectrum. J Neuro-Ophthalmol. 2019;39:391–3.
Alayoubi AM, Wang JCM, Au BCY, Carpentier S, Garcia V, Dworski S, et al. Systemic ceramide accumulation leads to severe and varied pathological consequences. EMBO Mol Med. 2013;5:827–42.
Dworski S, Berger A, Furlonger C, Moreau JM, Yoshimitsu M, Trentadue J, et al. Markedly perturbed hematopoiesis in acid ceramidase deficient mice. Haematologica. 2015;100:e162–e165.
Yu FPS, Sajdak BS, Sikora J, Salmon AE, Nagree MS, Gurka J, et al. Acid Ceramidase Deficiency in Mice Leads to Severe Ocular Pathology and Visual Impairment. Am J Pathol. 2019;189:320–38.
Barak A, Goldkorn T, Morse LS. Laser induces apoptosis and ceramide production in human retinal pigment epithelial cells. Investig Ophthalmol Vis Sci. 2005;46:2587–91.
Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: An overview and current perspectives. Biochim et Biophys Acta Mol Cell Biol Lipids. 2002;1585:114–25.
Ranty ML, Carpentier S, Cournot M, Rico-Lattes I, Malecaze F, Levade T, et al. Ceramide production associated with retinal apoptosis after retinal detachment. Graefe’s Arch Clin Exp Ophthalmol. 2009;247:215–24.
Lou H, Kang D, Yang Q, Lian C, Zhang C, Li Z, et al. Erythropoietin Protects Retina Against Ceramide 2-Induced Damage in Rat. Curr Mol Med. 2018;17:699–706.
Strettoi E, Gargini C, Sala G, Piano I, Gasco P, Ghidoni R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci USA 2010;107:18706–11.
Simón MV, Spalm Prado, Vera FH, Rotstein MS. N. P. Sphingolipids as emerging mediators in retina degeneration. Front Cell Neurosci. 2019;13:246.
Haddad S, Chen CA, Santangelo SL, Seddon JM. The Genetics of Age-Related Macular Degeneration: A Review of Progress to Date. Surv Ophthalmol. 2006;51:316–63.
He X, Schuchman EH. Ceramide and Ischemia/Reperfusion Injury. J Lipids 2018;2018:1–11.
Sanvicens N, Cotter TG. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem. 2006;98:1432–44.
Sugano E, Edwards G, Saha S, Wilmott LA, Grambergs RC, Mondal K, et al. Overexpression of acid ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress. J Lipid Res. 2019;60:30–43.
Opreanu M, Lydic TA, Reid GE, McSorley KM, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acid. Investig Ophthalmo Vis Sci. 2010;51:3253–63.
Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK. Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science. 2003;299:1740–3.
Fan J, Wu BX, Crosson CE. Suppression of acid sphingomyelinase protects the retina from ischemic injury. Investig Ophthalmol Vis Sci. 2016;57:4476–84.
Stiles M, Qi H, Sun E, Tan J, Porter H, Allegood J, et al. Sphingolipid profile alters in retinal dystrophic P23H-1 rats and systemic FTY720 can delay retinal degeneration. J Lipid Res. 2016;57:818–31.
Klein R, Klein BEK, Jensen SC, Cruickshanks KJ, Lee KE, Danforth L, et al. Medication use and the 5-year incidence of early age-related maculopathy: The Beaver Dam eye study. Arch Ophthalmol. 2001;119:1354–9.
He X, Dworski S, Zhu C, DeAngelis V, Solyom A, Medin JA, et al. Enzyme replacement therapy for Farber disease: Proof-of-concept studies in cells and mice. BBA Clin. 2017;7:85–96.
Garbade SF, Zielonka M, Mechler K, Kölker S, Hoffmann GF, Staufner C, et al. FDA orphan drug designations for lysosomal storage disorders - A cross-sectional analysis. PLoS One. 2020;15:e0230898.
Lachmann RH. Enzyme replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2011;23:588–93.
Brooks DA, Kakavanos R, Hopwood JJ. Significance of immune response to enzyme-replacement therapy for patients with a lysosomal storage disorder. Trend Mol Med. 2003;9:450–3.
Concolino D, Deodato F, Parini R. Enzyme replacement therapy: Efficacy and limitations. Ital J Pediatr. 2018;44:117–26.
Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21:3–9.
Rastall DPW, Amalfitano A. Recent advances in gene therapy for lysosomal storage disorders. Appl Clin Genet. 2015;8:157–69.
Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic dna with hot sodium hydroxide and tris (HotSHOT). Biotechniques. 2000;29:52–54.
Reid CA, Lipinski DM. Small and micro-scale recombinant adeno-associated virus production and purification for ocular gene therapy applications. Ret Gene Ther. 2018;1715:19–31.
McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–56.
Li Y, Benitez BA, Nagree MS, Dearborn JT, Jiang X, Guzman MA, et al. Genetic ablation of acid ceramidase in Krabbe disease confirms the psychosine hypothesis and identifies a new therapeutic target. Proc Natl Acad Sci USA 2019;116:20097–103.
Reid CA, Ertel KJ, Lipinski DM. Improvement of Photoreceptor Targeting via Intravitreal Delivery in Mouse and Human Retina Using Combinatory rAAV2 Capsid Mutant Vectors. Investg Ophthalmol Vis Sci. 2017;58:6429–39.
Piedra J, Ontiveros M, Miravet S, Penalva C, Monfar M, Chillon M. Development of a rapid, robust, and universal PicoGreen-based method to titer adeno-associated vectors. Hum Gene Ther Methods. 2015;26:35–42.
Zhang H, Sajdak BS, Merriman DK, McCall MA, Carroll J, Lipinski. Daniel M. Electroretinogram of the cone-dominant thirteen-lined ground squirrel during Euthermia and Hibernation in comparison with the rod-dominant Brown Norway rat. Investig Ophthalmol Vis Sci. 2020;61:6–6.
Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM. Fixation of testes and eyes using a modified Davidson’s fluid: Comparison with Bouin’s fluid and conventional Davidson’s fluid. Toxicol Pathol. 2002;30:524–33.
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb Protoc. 2008;5:pdb–prot4986.
Wolman M. The lipids stained by the periodic acid-schiff technic. Biotech Histochem. 1956;31:241–5.
Brannigan JA, Dodson G, Duggleby HJ, Moody PCE, Smith JL, Tomchick DR, et al. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature. 1995;378:413–6.
Shtraizent N, Eliyahu E, Park JH, He X, Shalgi R, Schuchman EH. Autoproteolytic cleavage and activation of human acid ceramidase. J Biol Chem. 2008;283:11253–9.
Abreu Velez AM, Upegui Zapata YA, Howard MS. Periodic acid-schiff staining parallels the immunoreactivity seen by direct immunofluorescence in autoimmune skin diseases. N. Am J Med Sci. 2016;8:151–5.
Winchester B. Lysosomal metabolism of glycoproteins. Glycobiology. 2005;15:1R–15R.
Adeva-Andany MM, González-Lucán M, Donapetry-García C, Fernández-Fernández C, Ameneiros-Rodríguez E. Glycogen metabolism in humans. BBA Clin. 2016;5:85–100.
Sandhoff K, Kolter T. Topology of glycosphingolipid degradation. Trends Cell Biol. 1996;6:98–103.
Chalfant CE, Kishikawal K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–7.
Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5:105–21.
Walvoort HC, Dormans JAMA, van den Ingh TSGAM. Comparative pathology of the canine model of glycogen storage disease type II (Pompe’s disease). J Inherit Metab Dis. 1985;8:38–46.
Pena LDM, Proia AD, Kishnani PS. Postmortem findings and clinical correlates in individuals with infantile-Onset pompe disease. JIMD Rep. 2015;23:45–54.
Solovyeva VV, Shaimardanova AA, Chulpanova DS, Kitaeva KV, Chakrabarti L, Rizvanov, AA. New approaches to Tay-Sachs disease therapy. Front Physiol. 2018;9:1663.
Baris HN, Cohen IJ, Mistry PK. Gaucher disease: The metabolic defect, pathophysiology, phenotypes and natural history. Pediatr Endocrinol Rev. 2014;12:72–81.
Allen MJ, Myer BJ, Khokher AM, Rushton N, Cox TM. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: Increased release of interleukin-6 and interleukin-10. QJM Mon J Assoc Phys. 1997;90:19–25.
Barak V, Acker M, Nisman B, Kalickman I, Abrahamov A, Zimran A, et al. Cytokines in Gaucher’s disease. Eur Cytokine Netw. 1999;10:205–10.
Echevarria FD, Formichella CR, Sappington RM. Interleukin-6 deficiency attenuates retinal ganglion cell axonopathy and glaucoma-related vision loss. Front Neurosci. 2017;11:318.
Wooff Y, Man SM, Aggio-Bruce R, Natoli R, Fernando N. IL-1 family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front Immunol. 2019;10:1618.
Okino N, He X, Gatt S, Sandhoff K, Ito M, Schuchman EH. The reverse activity of human acid ceramidase. J Biol Chem. 2003;278:29948–53.
Sikora J, Dworski S, Jones EE, Kamani MA, Micsenyi MC, Sawada T, et al. Acid Ceramidase Deficiency in Mice Results in a Broad Range of Central Nervous System Abnormalities. Am J Pathol. 2017;187:864–83.
Krishnamurthy K, Dasgupta S, Bieberich E. Development and characterization of a novel anti-ceramide antibody. J. Lipid Res. 2007;48:968–75.
Asanad S, Karanjia R. Full-Field Electroretinogram. Electrophysiology of Vision. 2022;4:1–64.
Almárcegui C. Pattern electroretinogram in anterior ischemic optic neuropathy. Rev Neurol. 2001;32:18–21.
Froehlich J, Kaufman DI. Use of pattern electroretinography to differentiate acute optic neuritis from acute anterior ischemic optic neuropathy. Electroencephalogr Clin Neurophysiol Evoked Potentials. 1994;92:480–6.
Bach M, Unsoeld AS, Philippin H, Staubach F, Maier P, Walter HS, et al. Pattern ERG as an early glaucoma indicator in ocular hypertension: A long-term, prospective study. Investig Ophthalmol Vis Sci. 2006;47:4881–7.
Giugliani R, Vairo F, Kubaski F, Poswar F, Riegel M, Baldo G, et al. J. A. Neurological manifestations of lysosomal disorders and emerging therapies targeting the CNS. Lancet Child Adolescent Health. 2018;2:56–68.
Acknowledgements
The authors thank Lisa King, Christine Skumatz, Joseph Thulin, and the Biomedical Resource Center at MCW for their help with animal care, in addition to Christine Duris, Qiuhui Yang, and the Children’s Research Institute histology core of MCW for their contributions to histological studies. We thank Benyapa Khowpinitchai and Alexander Salmon for their help on OCT data measurement and analysis. We thank Xuntian Jiang and the Metabolomics Core at Washington University (St. Louis, MO) for assistance with sphingolipid quantification.
Funding
This study was supported through intramural funds available to DML and JAM.
Author information
Authors and Affiliations
Contributions
HZ and MSN designed, conducted the experiments, and wrote the manuscript; HL and XP performed OCT data analysis and statistics; JAM supervised the study and paper review; DML designed the experiments, conducted the mice injection, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Nagree, M.S., Liu, H. et al. rAAV-mediated over-expression of acid ceramidase prevents retinopathy in a mouse model of Farber lipogranulomatosis. Gene Ther 30, 297–308 (2023). https://doi.org/10.1038/s41434-022-00359-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-022-00359-w