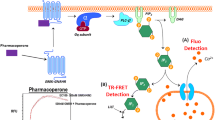
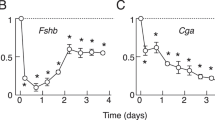
Abstract
The reproductive axis is activated by gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gonadotropes to secrete hormones that drive gonadal function and steroidogenesis. Thus repression of this axis, which is conserved across mammals and sexes, can reduce steroid levels and/or prevent reproduction. Steroid-dependent pathologies, including various cancers, are commonly treated with GnRH super-analogs which have long-term side-effects, while humane solutions for controlling reproduction in domestic and wild animal populations are lacking. GnRH-conjugated toxins are undergoing clinical trials for GnRHR-expressing cancer cells, and have been examined for gonadotrope ablation in animals, but showed low and/or transient effects and administration of toxins has many potential complications. Here we exploit GnRH targeting to gonadotropes to deliver DNA encoding an effector that induces gonadotropin gene repressive epigenetic modifications which are perpetuated over time. Several layers of specificity are endowed through targeting to GnRHR-expressing cells and due to local cleavage of the peptide packaging the DNA; the DNA-encoded effector is expressed and directed to the target genes by the DNA binding domain of a highly specific transcription factor. This design has multiple advantages over existing methods of shutting down the reproductive axis, and its modular design should allow adaptation for broad applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data generated is contained in the manuscript and/or Supplementary Materials.
References
Suo L, Chang X, Xu N, Ji H. The anti-proliferative activity of GnRH through downregulation of the Akt/ERK pathways in pancreatic cancer. Front Endocrinol. 2019;10:370.
Gründker C, Ernst J, Reutter M, Ghadimi BM, Emons G. Effective targeted chemotherapy using AEZS-108 (AN-152) for LHRH receptor-positive pancreatic cancers. Oncol Rep. 2011;26:629–35.
Maggi R, Cariboni AM, Marelli MM, Moretti RM, Andrè V, Marzagalli M, et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum Reprod Update. 2016;22:358–81.
Gründker C, Emons G. The role of gonadotropin-releasing hormone in cancer cell proliferation and metastasis. Front Endocrinol. 2017;8:187.
Föst C, Duwe F, Hellriegel M, Schweyer S, Emons G, Gründker C. Targeted chemotherapy for triple-negative breast cancers via LHRH receptor. Oncol Rep. 2011;25:1481–7.
Seitz S, Buchholz S, Schally AV, Weber F, Klinkhammer-Schalke M, Inwald EC, et al. Triple negative breast cancers express receptors for LHRH and are potential therapeutic targets for cytotoxic LHRH-analogs, AEZS 108 and AEZS 125. BMC Cancer. 2014;14:847.
Kwok CW, Treeck O, Buchholz S, Seitz S, Ortmann O, Engel JB. Receptors for luteinizing hormone-releasing hormone (GnRH) as therapeutic targets in triple negative breast cancers (TNBC). Target Oncol. 2015;10:365–73.
Li X, Taratula OO, Taratula OO, Schumann C, Minko T. LHRH-targeted drug delivery systems for cancer therapy. Mini Rev Med Chem. 2016;17:258–67.
Limonta P, Marelli MM, Mai S, Motta M, Martini L, Moretti RM. GnRH receptors in cancer: from cell biology to novel targeted therapeutic strategies. Endocr Rev. 2012;33:784–811.
Engel JB, Tinneberg H-R, Rick FG, Berkes E, Schally AV. Targeting of peptide cytotoxins to LHRH receptors for treatment of cancer. Curr Drug Targets. 2016;17:488–94.
Fontana F, Marzagalli M, Marelli MM, Raimondi M, Moretti RM, Limonta P. Gonadotropin-releasing hormone receptors in prostate cancer: molecular aspects and biological functions. Int J Mol Sci. MDPI AG. 2020;21:1–23.
Westphalen S, Kotulla G, Kaiser F, Krauss W, Werning G, Elsasser HP, et al. Receptor mediated antiproliferative effects of the cytotoxic LHRH agonist AN-152 in human ovarian and endometrial cancer cell lines. Int J Oncol. 2000;17:1063–9.
Miller DS, Scambia G, Bondarenko I, Westermann AM, Oaknin A, Oza AM, et al. ZoptEC: Phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer (NCT01767155). J Clin Oncol. 2018;36:5503–5503.
Curtis KK, Sarantopoulos J, Northfelt DW, Weiss GJ, Barnhart KM, Whisnant JK, et al. Novel LHRH-receptor-targeted cytolytic peptide, EP-100: First-in-human phase I study in patients with advanced LHRH-receptor-expressing solid tumors. Cancer Chemother Pharmacol. 2014;73:931–41.
Davenport AP, Scully CCG, de Graaf C, Brown AJH, Maguire JJ. Advances in therapeutic peptides targeting G protein-coupled receptors. Nat Rev Drug Discov. 2020;19:389–413.
Ball BA, Sabeur K, Nett T, Liu IKM. Effects of a GnRH cytotoxin on reproductive function in peripubertal male dogs. Theriogenology. 2006;66:766–74.
Sabeur K, Ball BA, Nett TM, Ball HH, Liu IKM. Effect of GnRH conjugated to pokeweed antiviral protein on reproductive function in adult male dogs. Reproduction. 2003;125:801–6.
Nagy A, Schally AV, Armatis P, Szepeshazi K, Halmos G, Kovacs M, et al. Cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolinodoxorubicin, a derivative 500-1000 times more potent. Proc Natl Acad Sci USA. 1996;93:7269–73.
Nagy A, Plonowski A, Schally AV. Stability of cytotoxic luteinizing hormone-releasing hormone conjugate (AN-152) containing doxorubicin 14-O-hemiglutarate in mouse and human serum in vitro: Implications for the design of preclinical studies. Proc Natl Acad Sci USA. 2000;97:829–34.
Struthers RS. Gonadotropin-releasing hormone targeting for gonadotroph ablation: an approach to non-surgical sterilization. Reprod Domest Anim. 2012;47:233–8.
Kovacs M, Schally AV, Nagy A, Koppan M, Groot K. Recovery of pituitary function after treatment with a targeted cytotoxic analog of luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA. 1997;94:1420–5.
Kovacs M, Schally AV, Csernus B, Busto R, Rekasi Z, Nagy A. Targeted cytotoxic analogue of luteinizing hormone-releasing hormone (LH-RH) only transiently decreases the gene expression of pituitary receptors for LH-RH. J Neuroendocrinol. 2002;14:5–13.
Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309.
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–64.
Dawson MA. The cancer epigenome: concepts, challenges, and therapeutic opportunities. Science. 2017;355:1147–52.
Gilan O, Rioja I, Knezevic K, Bell MJ, Yeung MM, Harker NR, et al. Selective targeting of BD1 and BD2 of the BET proteins in cancer and immunoinflammation. Science. 2020;368:387–94.
Ayyanathan K, Lechner MS, Bell P, Maul GG, Schultz DC, Yamada Y, et al. Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 2003;17:1855–69.
Alerasool N, Segal D, Lee H, Taipale M. An efficient KRAB domain for CRISPRi applications in human cells. Nat Methods. 2020;17:1093–6. https://pubmed.ncbi.nlm.nih.gov/33020655/
O’Geen H, Bates SL, Carter SS, Nisson KA, Halmai J, Fink KD, et al. Ezh2-dCas9 and KRAB-dCas9 enable engineering of epigenetic memory in a context-dependent manner. Epigenet Chromatin. 2019;12:26.
Miranda Furtado CL, Dos Santos Luciano MC, Silva Santos R Da, Furtado GP, Moraes MO, et al. Epidrugs: targeting epigenetic marks in cancer treatment. Epigenetics. 2019;14:1164–76.
Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19:776–800.
Wimalasena VK, Wang T, Sigua LH, Durbin AD, Qi J. Using chemical epigenetics to target cancer. Mol Cell Cell Press. 2020;78:1086–95.
Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19:673–94.
Rudnizky S, Khamis H, Malik O, Melamed P, Kaplan A. The base pair-scale diffusion of nucleosomes modulates binding of transcription factors. Proc Natl Acad Sci USA. 2019;116:12161–6.
Rudnizky S, Bavly A, Malik O, Pnueli L, Melamed P, Kaplan A. H2A.Z controls the stability and mobility of nucleosomes to regulate expression of the LH genes. Nat Commun. 2016;7. https://pubmed.ncbi.nlm.nih.gov/27653784/.
Yosefzon Y, David C, Tsukerman A, Pnueli L, Qiao S, Boehm U, et al. An epigenetic switch repressing Tet1 in gonadotropes activates the reproductive axis. Proc Natl Acad Sci USA. 2017;114:10131–6.
Wijeweera A, Haj M, Feldman A, Pnueli L, Luo Z, Melamed P. Gonadotropin gene transcription is activated by menin-mediated effects on the chromatin. Biochim Biophys Acta. 2015;1849:328–41.
Haj M, Wijeweera A, Rudnizky S, Taunton J, Pnueli L, Melamed P. Mitogen- and stress-activated protein kinase 1 is required for gonadotropin-releasing hormone-mediated activation of gonadotropin α-subunit expression. J Biol Chem. 2017;292:20720–31.
Melamed P. Histone deacetylases and repression of the gonadotropin genes. Trends Endocrinol Metab. 2008;19:25–31.
Lim S, Luo M, Koh M, Yang M, bin Abdul Kadir MN, Tan JH, et al. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin -subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27:4105–20.
Shalev D, Melamed P. The role of the hypothalamus and pituitary epigenomes in central activation of the reproductive axis at puberty. Mol Cell Endocrinol. 2020;518:111031.
Melamed P, Haj M, Yosefzon Y, Rudnizky S, Wijeweera A, Pnueli L, et al. Multifaceted targeting of the chromatin mediates gonadotropin-releasing hormone effects on gene expression in the gonadotrope. Front Endocrinol. 2018;9:58 http://www.ncbi.nlm.nih.gov/pubmed/29535683
Glover DJ, Ng SM, Mechler A, Martin LL, Jans DA. Multifunctional protein nanocarriers for targeted nuclear gene delivery in nondividing cells. FASEB J. 2009;23:2996–3006.
Ekladious I, Colson YL, Grinstaff MW. Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov. 2019;18:273–94.
Mains RE, Berard CA, Denault JB, Zhou A, Johnson RC, Leduc R. PACE4: a subtilisin-like endoprotease with unique properties. Biochem J. 1997;321:587–93.
Kurrikoff K, Freimann K, Veiman KL, Peets EM, Piirsoo A, Langel Ü. Effective lung-targeted RNAi in mice with peptide-based delivery of nucleic acid. Sci Rep. 2019;9:19926.
Carreras-Badosa G, Maslovskaja J, Periyasamy K, Urgard E, Padari K, Vaher H, et al. NickFect type of cell-penetrating peptides present enhanced efficiency for microRNA-146a delivery into dendritic cells and during skin inflammation. Biomaterials. 2020;262:120316.
Liu BR, Huang YW, Aronstam RS, Lee HJ. Identification of a short cell-penetrating peptide from bovine lactoferricin for intracellular delivery of DNA in human A549 cells. PLoS One. 2016;11:e0150439.
Kurrikoff K, Veiman KL, Künnapuu K, Peets EM, Lehto T, Pärnaste L, et al. Effective in vivo gene delivery with reduced toxicity, achieved by charge and fatty acid -modified cell penetrating peptide. Sci Rep. 2017;7:17056.
Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using nRH receptor-expressing pituitary cell lines*. Endocr Rev. 1997;18:46–70.
Hazum E, Cuatrecasas P, Marian J, Conn PM. Receptor-mediated internalization of fluorescent gonadotropin-releasing hormone by pituitary gonadotropes. Proc Natl Acad Sci USA. 1980;77:6692–5.
Horn F, Bilezikjian LM, Perrin MH, Bosma MM, Windle JJ, Huber KS, et al. Intracellular responses to gonadotropin-releasing hormone in a clonal cell line of the gonadotrope lineage. Mol Endocrinol. 1991;5:347–55.
Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, et al. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–54.
Jorgensen JS, Quirk CC, Nilson JH. Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev. 2004;25:521–42.
Pnueli L, Luo M, Wang S, Naor Z, Melamed P. Calcineurin mediates the gonadotropin-releasing hormone effect on expression of both subunits of the follicle-stimulating hormone through distinct mechanisms. Mol Cell Biol. 2011;31:5023–36.
Sadie H, Styger G, Hapgood J. Expression of the mouse gonadotropin-releasing hormone receptor gene in αT3-1 gonadotrope cells is stimulated by cyclic 3′,5′-adenosine monophosphate and protein kinase A, and is modulated by steroidogenic factor-1 and Nur77. Endocrinology. 2003;144:1958–71.
Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Mol Cell Biol. 1999;19:2567–76.
Kaiser UB, Halvorson LM, Chen MT. Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (Egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-β gene promoter: an integral role for SF-1. Mol Endocrinol. 2000;14:1235–45.
Melamed P, Zhu Y, Siew HTHT, Xie M, Koh M. Gonadotropin-releasing hormone activation of C-jun, but not early growth response factor-1, stimulates transcription of a luteinizing hormone β-subunit gene. Endocrinology. 2006;147:3598–605.
Feng J, Lawson M, Melamed P. A proteomic comparison of immature and mature mouse gonadotrophs reveals novel differentially expressed nuclear proteins that regulate gonadotropin gene transcription and RNA splicing. Biol Reprod. 2021;79:546–61. https://pubmed.ncbi.nlm.nih.gov/18480465/
Ying Y, Yang X, Zhao K, Mao J, Kuang Y, Wang Z, et al. The Krüppel-associated box repressor domain induces reversible and irreversible regulation of endogenous mouse genes by mediating different chromatin states. Nucleic Acids Res. 2015;43:1549–61.
Hammond SM, Aartsma‐Rus A, Alves S, Borgos SE, Buijsen RAM, Collin RWJ, et al. Delivery of oligonucleotide‐based therapeutics: challenges and opportunities. EMBO Mol Med. 2021;13:e13243.
Matharu N, Ahituv N. Modulating gene regulation to treat genetic disorders. Nat Rev Drug Discov. 2020;19:757–75. Volp
Dissen GA, Adachi K, Lomniczi A, Chatkupt T, Davidson BL, Nakai H, et al. Engineering a gene silencing viral construct that targets the cat hypothalamus to induce permanent sterility: an update. Reprod Domest Anim. 2017;52:354–8. Apr
Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci. 2017;38:406–24.
Jauset T, Beaulieu ME. Bioactive cell penetrating peptides and proteins in cancer: a bright future ahead. Curr Opin Pharmacol. 2019;47:133–40.
Xie J, Bi Y, Zhang H, Dong S, Teng L, Lee RJ, et al. Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application. Front Pharmacol. 2020;11:697.
Mullard A. Gene-editing pipeline takes off. Nat Rev Drug Discov. 2020;19:367–72. Volp
Kurrikoff K, Langel Ü. Recent CPP-based applications in medicine. Expert Opin Drug Del. 2019;16:1183–91.
Saar K, Lindgren M, Hansen M, Eiríksdóttir E, Jiang Y, Rosenthal-Aizman K, et al. Cell-penetrating peptides: a comparative membrane toxicity study. Anal Biochem. 2005;345:55–65.
Arukuusk P, Pärnaste L, Oskolkov N, Copolovici DM, Margus H, Padari K, et al. New generation of efficient peptide-based vectors, NickFects, for the delivery of nucleic acids. Biochim Biophys Acta Biomembr. 2013;1828:1365–73.
Maenhoudt C, Santos N, Fontbonne A. Suppression of fertility in adult dogs. Reprod Domest Anim. 2014;49:58–63.
Massei G, Miller LA. Nonsurgical fertility control for managing free-roaming dog populations: a review of products and criteria for field applications. Theriogenol Theriogenol. 2013;80:829–38.
Goericke-Pesch S, Wehrend A, Georgiev P. Suppression of fertility in adult cats. Reprod Domest Anim. 2014;49:33–40. SUPPL.2
Munks MW. Progress in development of immunocontraceptive vaccines for permanent non-surgical sterilization of cats and dogs. Reprod Domest Anim. 2012;47:223–7.
Fischer A, Benka VAW, Briggs JR, Driancourt MA, Maki J, Mora DSO, et al. Effectiveness of GonaCon as an immunocontraceptive in colony-housed cats. J Feline Med Surg. 2018;20:786–92.
DiGangi BA, Grijalva J, Jaramillo EPP, Dueñas I, Glenn C, Cruz MEC, et al. Post-operative outcomes of surgical and chemical castration with zinc gluconate in dogs presenting to veterinary field clinics. Vet J. 2017;229:26–30.
Hay BA, Li J, Guo M. Vectored gene delivery for lifetime animal contraception: overview and hurdles to implementation. Theriogenology. 2018;112:63–74. May
Johnson AK, Jones RL, Kraneburg CJ, Cochran AM, Samoylov AM, Wright JC, et al. Phage constructs targeting gonadotropin-releasing hormone for fertility control: evaluation in cats. J Feline Med Surg. 2020;22:685–95. Aug
Dissen GA, Lomniczi A, Boudreau RL, Chen YH, Davidson BL, Ojeda SR. Targeted gene silencing to induce permanent sterility. Reprod Domest Anim. 2012;47:228–32.
Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15:712–29.
Funding
This research was supported by the Office of the Chief Scientist, Ministry of Agriculture (14-16-0004), POLAK Fund for Applied Research and Hittman Family Foundation Biomedical Innovation Fund.
Author information
Authors and Affiliations
Contributions
LP and PM conceived the project, analyzed the results and wrote the manuscript. LP performed the experiments. PM acquired the funding.
Corresponding author
Ethics declarations
Competing interests
LP and PM are inventors on US Provisional Patent Application No. 63/221,056.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pnueli, L., Melamed, P. Epigenetic repression of gonadotropin gene expression via a GnRH-mediated DNA delivery system. Gene Ther 29, 294–303 (2022). https://doi.org/10.1038/s41434-022-00325-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-022-00325-6