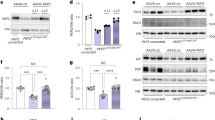
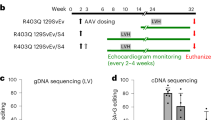
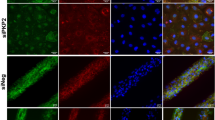
Abstract
Ischemic cardiomyopathy is a leading cause of death and an unmet clinical need. Adeno-associated virus (AAV) gene-based therapies hold great promise for treating and preventing heart failure. Previously we showed that muscle A-kinase Anchoring Protein β (mAKAPβ, AKAP6β), a scaffold protein that organizes perinuclear signalosomes in the cardiomyocyte, is a critical regulator of pathological cardiac hypertrophy. Here, we show that inhibition of mAKAPβ expression in stressed adult cardiomyocytes in vitro was cardioprotective, while conditional cardiomyocyte-specific mAKAP gene deletion in mice prevented pathological cardiac remodeling due to myocardial infarction. We developed a new self-complementary serotype 9 AAV gene therapy vector expressing a short hairpin RNA for mAKAPβ under the control of a cardiomyocyte-specific promoter (AAV9sc.shmAKAP). This vector efficiently downregulated mAKAPβ expression in the mouse heart in vivo. Expression of the shRNA also inhibited mAKAPβ expression in human induced cardiomyocytes in vitro. Following myocardial infarction, systemic administration of AAV9sc.shmAKAP prevented the development of pathological cardiac remodeling and heart failure, providing long-term restoration of left ventricular ejection fraction. Our findings provide proof-of-concept for mAKAPβ as a therapeutic target for ischemic cardiomyopathy and support the development of a translational pipeline for AAV9sc.shmAKAP for the treatment of heart failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Additional data and original echocardiographic images are available from the corresponding author on reasonable request.
References
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 update: a report From the American Heart Association. Circulation. 2021;143:e254–e743.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407.
Omar MH, Scott JD. AKAP signaling islands: venues for precision pharmacology. Trends Pharmacol Sci. 2020;41:933–46.
Negro A, Dodge-Kafka K, Kapiloff MS. Signalosomes as therapeutic targets. Prog Pediatr Cardiol. 2008;25:51–6.
Zaccolo M, Zerio A, Lobo MJ. Subcellular organization of the cAMP signaling pathway. Pharmacol Rev. 2021;73:278–309.
Li J, Tan Y, Passariello CL, Martinez EC, Kritzer MD, Li X, et al. Signalosome-regulated serum response factor phosphorylation determining myocyte growth in width versus length as a therapeutic target for heart failure. Circulation. 2020;142:2138–54.
Li X, Li J, Martinez EC, Froese A, Passariello CL, Henshaw K, et al. Calcineurin Abeta-specific anchoring confers isoform-specific compartmentation and function in pathological cardiac myocyte hypertrophy. Circulation. 2020;142:948–62.
Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, et al. The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ Heart Fail. 2014;7:663–72.
Kieserman JM, Myers VD, Dubey P, Cheung JY, Feldman AM. Current landscape of heart failure gene therapy. J Am Heart Assoc. 2019;8:e012239.
Dodge-Kafka K, Gildart M, Tokarski K, Kapiloff MS. mAKAPbeta signalosomes–A nodal regulator of gene transcription associated with pathological cardiac remodeling. Cell Signal. 2019;63:109357.
Li J, Vargas MA, Kapiloff MS, Dodge-Kafka KL. Regulation of MEF2 transcriptional activity by calcineurin/mAKAP complexes. Exp Cell Res. 2013;319:447–54.
Li J, Negro A, Lopez J, Bauman AL, Henson E, Dodge-Kafka K, et al. The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol. 2010;48:387–94.
Dodge-Kafka KL, Gildart M, Li J, Thakur H, Kapiloff MS. Bidirectional regulation of HDAC5 by mAKAPbeta signalosomes in cardiac myocytes. J Mol Cell Cardiol. 2018;118:13–25.
Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal. 2008;1:ra18.
Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–5.
Prasad KM, Xu Y, Yang Z, Acton ST, French BA. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 2011;18:43–52.
Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ, et al. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet. 2005;37:1281–8.
Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–11.
Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005;118:5637–46.
Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci. 1999;112:2725–36.
Feyen DAM, Perea-Gil I, Maas RGC, Harakalova M, Gavidia AA, Arthur Ataam J, et al. Unfolded protein response as a compensatory mechanism and potential therapeutic target in PLN R14del Cardiomyopathy. Circulation. 2021;144:382–92.
Kuzmin DA, Shutova MV, Johnston NR, Smith OP, Fedorin VV, Kukushkin YS, et al. The clinical landscape for AAV gene therapies. Nat Rev Drug Discov. 2021;20:173–4.
Konkalmatt PR, Wang F, Piras BA, Xu Y, O’Connor DM, Beyers RJ, et al. Adeno-associated virus serotype 9 administered systemically after reperfusion preferentially targets cardiomyocytes in the infarct border zone with pharmacodynamics suitable for the attenuation of left ventricular remodeling. J Gene Med. 2012;14:609–20.
Vergarajauregui S, Becker R, Steffen U, Sharkova M, Esser T, Petzold J, et al. AKAP6 orchestrates the nuclear envelope microtubule-organizing center by linking golgi and nucleus via AKAP9. Elife. 2020;9:e61669.
Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–50.
Fraccarollo D, Galuppo P, Bauersachs J. Novel therapeutic approaches to post-infarction remodelling. Cardiovasc Res. 2012;94:293–303.
Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–75.
Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8.
Sam F, Sawyer DB, Chang DL, Eberli FR, Ngoy S, Jain M, et al. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol Heart Circ Physiol. 2000;279:H422–8.
Abbate A, Biondi-Zoccai GG, Bussani R, Dobrina A, Camilot D, Feroce F, et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41:753–60.
Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, et al. Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res. 2004;94:91–9.
Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–41.
Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, et al. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem. 2008;283:22295–303.
Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, et al. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol. 2002;22:7603–13.
Kim Y, Phan D, van Rooij E, Wang DZ, McAnally J, Qi X, et al. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest. 2008;118:124–32.
Li J, Aponte Paris S, Thakur H, Kapiloff MS, Dodge-Kafka KL. Muscle A-kinase-anchoring protein-beta-bound calcineurin toggles active and repressive transcriptional complexes of myocyte enhancer factor 2D. J Biol Chem. 2019;294:2543–54.
Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, et al. Phospholipase Cepsilon hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell. 2013;153:216–27.
Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, Ahn J, et al. cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem. 2010;285:11078–86.
Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res. 2005;303:388–99.
Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–71.
Funding
This work was supported by NIH Grants R01HL126825, R01HL153835, and R01HL146111 (Dr. Kapiloff and Dr. Dodge-Kafka) and the NHLBI Gene Therapy Resource Program.
Author information
Authors and Affiliations
Contributions
ECM performed in vivo mouse research and analysis of primary data with the assistance of JL and HT. JAA performed human induced cardiomyocyte experiments. KT performed rat cardiomyocyte experiments. MSK, ECM, IK, and KDK wrote the paper. MSK provided overall supervision for the project.
Corresponding author
Ethics declarations
Ethical approval
Animal research was approved by the Institutional Animal Care and Use Committee at the University of Miami and the University of Connecticut. The Stanford University Institutional Review Board approved the use of pluripotent stem cells.
Competing interests
Drs. Kapiloff and Li are inventors of patent-protected intellectual property concerning the targeting of mAKAPβ signalosomes to treat heart failure, by which they, the University of Miami, and Stanford University may gain royalties from future commercialization. Dr. Kapiloff holds equity in Anchored RSK3 Inhibitors, LLC, and Cardiac RSK3 Inhibitors, LLC, companies interested in developing mAKAP signalosome-targeted therapies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Martinez, E.C., Li, J., Ataam, J.A. et al. Targeting mAKAPβ expression as a therapeutic approach for ischemic cardiomyopathy. Gene Ther 30, 543–551 (2023). https://doi.org/10.1038/s41434-022-00321-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-022-00321-w