Abstract
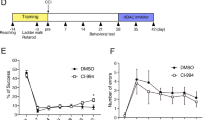
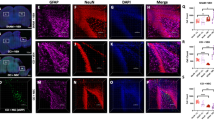
Traumatic brain injury (TBI) survivors suffer from long-term disability and neuropsychiatric sequelae due to irreparable brain tissue destruction. However, there are still few efficient therapies to promote neurorestoration in damaged brain tissue. This study aimed to investigate whether the pro-oncogenic gene ski can promote neurorestoration after TBI. We established a ski-overexpressing experimental TBI mouse model using adenovirus-mediated overexpression through immediate injection after injury. Hematoxylin-eosin staining, MRI-based 3D lesion volume reconstruction, neurobehavioral tests, and analyses of neuronal regeneration and astrogliosis were used to assess neurorestorative efficiency. The effects of ski overexpression on the proliferation of cultured immature neurons and astrocytes were evaluated using imaging flow cytometry. The Ski protein level increased in the perilesional region at 3 days post injury. ski overexpression further elevated Ski protein levels up to 14 days post injury. Lesion volume was attenuated by approximately 36–55% after ski overexpression, with better neurobehavioral recovery, more newborn immature and mature neurons, and less astrogliosis in the perilesional region. Imaging flow cytometry results showed that ski overexpression elevated the proliferation rate of immature neurons and reduced the proliferation rate of astrocytes. These results show that ski can be considered a novel neurorestoration-related gene that effectively promotes neurorestoration, facilitates neuronal regeneration, and reduces astrogliosis after TBI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
Data are available on request due to privacy/ethical restrictions. The data that support the findings of this study are available on request from the corresponding author.
References
Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–63.
McArthur D, Chute D, Villablanca J. Moderate and severe traumatic brain injury: epidemiologic, imaging and neuropathologic perspectives. Brain Pathol. 2004;14:185–94.
Stein M, Jain S, Giacino J, Levin H, Dikmen S, Nelson L, et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: a TRACK-TBI study. JAMA Psychiatry. 2019;76:249–58.
Baker EW, Kinder HA, Hutcheson JM, Duberstein KJJ, Platt SR, Howerth EW, et al. Controlled cortical impact severity results in graded cellular, tissue, and functional responses in a piglet traumatic brain injury model. J Neurotrauma. 2019;36:61–73.
Krucoff MO, Rahimpour S, Slutzky MW, Edgerton VR, Turner DA. Enhancing nervous system recovery through neurobiologics, neural interface training, and neurorehabilitation. Front Neurosci. 2016;10:584.
Neckel N, Dai H, Burns M. A novel multi-dimensional analysis of rodent gait reveals the compensation strategies used during spontaneous recovery from spinal cord and traumatic brain injury. J Neurotrauma. 2020;37:517–27.
Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab. 2013;33:524–31.
Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60.
Ngwenya LB, Danzer SC. Impact of traumatic brain injury on neurogenesis. Front Neurosci. 2018;12:1014.
Patel K, Sun D. Strategies targeting endogenous neurogenic cell response to improve recovery following traumatic brain injury. Brain Res. 2016;1640:104–13.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35.
Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA. 2005;102:8333–8.
Witcher K, Bray C, Dziabis J, McKim D, Benner B, Rowe R, et al. Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation. Glia. 2018;66:2719–36.
Xiong Y, Mahmood A, Chopp M. Neurorestorative treatments for traumatic brain injury. Discov Med. 2010;10:434–42.
Maas A, Roozenbeek B, Manley G. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7:115–26.
Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao L. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26:1118–30.
Li Y, Turck CM, Teumer JK, Stavnezer E. Unique sequence, ski, in Sloan-Kettering avian retroviruses with properties of a new cell-derived oncogene. J Virol. 1986;57:1065–72.
Liu X, Li P, Liu P, Xiong R, Zhang E, Chen X, et al. The essential role for c-Ski in mediating TGF-beta1-induced bi-directional effects on skin fibroblast proliferation through a feedback loop. Biochem J. 2008;409:289–97.
Soeta C, Suzuki M, Suzuki S, Naito K, Tachi C, Tojo H. Possible role for the c-ski gene in the proliferation of myogenic cells in regenerating skeletal muscles of rats. Dev Growth Differ. 2001;43:155–64.
Macias-Silva M, Li W, Leu JI, Crissey MA, Taub R. Up-regulated transcriptional repressors SnoN and Ski bind Smad proteins to antagonize transforming growth factor-beta signals during liver regeneration. J Biol Chem. 2002;277:28483–90.
Reyes-Gordillo K, Shah R, Arellanes-Robledo J, Hernández-Nazara Z, Rincón-Sánchez AR, Inagaki Y, et al. Mechanisms of action of acetaldehyde in the up-regulation of the human α2(I) collagen gene in hepatic stellate cells: key roles of Ski, SMAD3, SMAD4, and SMAD7. Am J Pathol. 2014;184:1458–67.
Yamamoto T, Togawa A, Ohashi N, Fujigaki Y, Oda T, Uchida C, et al. Ubiquitin-dependent degradation of SnoN and Ski is increased in renal fibrosis induced by obstructive injury. Kidney Int. 2006;69:1733–40.
Zhang C, Zhang Y, Zhu H, Hu J, Xie Z. MiR-34a/miR-93 target c-Ski to modulate the proliferaton of rat cardiac fibroblasts and extracellular matrix deposition in vivo and in vitro. Cell Signal. 2018;46:145–53.
Bonnon C, Atanasoski S. c-Ski in health and disease. Cell Tissue Res. 2011;347:51–64.
Liu X, Li P, Chen XY, Zhou YG. c-Ski promotes skin fibroblast proliferation but decreases type I collagen: implications for wound healing and scar formation. Clin Exp Dermatol. 2010;35:417–24.
Li P, Liu P, Xiong RP, Chen XY, Zhao Y, Lu WP, et al. Ski, a modulator of wound healing and scar formation in the rat skin and rabbit ear. J Pathol. 2011;223:659–71.
Atanasoski S, Notterpek L, Lee HY, Castagner F, Young P, Ehrengruber MU, et al. The protooncogene Ski controls Schwann cell proliferation and myelination. Neuron. 2004;43:499–511.
Lyons GE, Micales BK, Herr MJ, Horrigan SK, Namciu S, Shardy D, et al. Protooncogene c-ski is expressed in both proliferating and postmitotic neuronal populations. Dev Dyn. 1994;201:354–65.
Baranek C, Atanasoski S. Modulating epigenetic mechanisms: the diverse functions of Ski during cortical development. Epigenetics. 2012;7:676–9.
Berk M, Desai SY, Heyman HC, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial patterning, and skeletal muscle development. Genes Dev. 1997;11:2029–39.
Colmenares C, Heilstedt HA, Shaffer LG, Schwartz S, Berk M, Murray JC, et al. Loss of the SKI proto-oncogene in individuals affected with 1p36 deletion syndrome is predicted by strain-dependent defects in Ski-/- mice. Nature Genet. 2002;30:106–9.
Kratzer R, Kreppel F. Production, purification, and titration of first-generation adenovirus vectors. Methods Mol Biol. 2017;1654:377–88.
An C, Jiang X, Pu H, Hong D, Zhang W, Hu X, et al. Severity-dependent long-term spatial learning-memory impairment in a mouse model of traumatic brain injury. Transl Stroke Res. 2016;7:512–20.
Wang G, Jiang X, Pu H, Zhang W, An C, Hu X, et al. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway: scriptaid protects against TBI via AKT. Neurotherapeutics. 2013;10:124–42.
Siddiq I, Park E, Liu E, Spratt S, Surosky R, Lee G, et al. Treatment of traumatic brain injury using zinc-finger protein gene therapy targeting VEGF-A. J Neurotrauma. 2012;29:2647–59.
Pan L, Shang N, Shangguan J, Figini M, Xing W, Wang B, et al. Magnetic resonance imaging monitoring therapeutic response to dendritic cell vaccine in murine orthotopic pancreatic cancer models. Am J Cancer Res. 2019;9:562–73.
Coutinho J, Ramos AF, Maia L, Castro L, Conceicao E, Geliebter A, et al. Volumetric alterations in the nucleus accumbens and caudate nucleus in bulimia nervosa: a structural magnetic resonance imaging study. Int J Eat Disord. 2015;48:206–14.
Petullo D, Masonic K, Lincoln C, Wibberley L, Teliska M, Yao D. Model development and behavioral assessment of focal cerebral ischemia in rats. Life Sci. 1999;64:1099–108.
Kim H, Yu T, Cam-Etoz B, van Groen T, Hubbard W, Chaudry I. Treatment of traumatic brain injury with 17α-ethinylestradiol-3-sulfate in a rat model. J Neurosurg. 2017;127:23–31.
Toshkezi G, Kyle M, Longo S, Chin L, Zhao L. Brain repair by hematopoietic growth factors in the subacute phase of traumatic brain injury. J Neurosurg. 2018;129:1286–94.
Zeng XJ, Li P, Ning YL, Zhao Y, Peng Y, Yang N, et al. Impaired autophagic flux is associated with the severity of trauma and the role of A2AR in brain cells after traumatic brain injury. Cell Death Dis. 2018;9:252.
Wang Y, Tang J, Xu X, Zhou X, Du J, Wang X, et al. NMDA receptors inhibit axonal outgrowth by inactivating Akt and activating GSK-3β via calcineurin in cultured immature hippocampal neurons. Exp Cell Res. 2018;371:389–98.
Kaur P, Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr Neuropharmacol. 2018;16:1224–38.
Lazaridis C, Rusin C, Robertson C. Secondary brain injury: predicting and preventing insults. Neuropharmacology. 2019;145:145–52.
Bramlett HM, Dietrich WD. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015;32:1834–48.
Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103:607–14.
Osier ND, Carlson SW, DeSana A, Dixon CE. Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J Neurotrauma. 2015;32:1861–82.
Urrea C, Castellanos D, Sagen J, Tsoulfas P, Bramlett H, Dietrich W. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76.
Villapol S, Byrnes KR, Symes AJ. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front Neurol. 2014;5:82.
Sebastiani A, Gölz C, Werner C, Schäfer M, Engelhard K, Thal S. Proneurotrophin binding to P75 neurotrophin receptor (P75ntr) is essential for brain lesion formation and functional impairment after experimental traumatic brain injury. J Neurotrauma. 2015;32:1599–607.
Sîrbulescu R, Chung J, Edmiston W, Poznansky S, Poznansky M, Whalen M. Intraparenchymal application of mature B lymphocytes improves structural and functional outcome after contusion traumatic brain injury. J Neurotrauma. 2019;36:2579–89.
Liu X, Zhang E, Li P, Liu J, Zhou P, Gu D, et al. Expression and possible mechanism of c-ski, a novel tissue repair-related gene during normal and radiation-impaired wound healing. Wound Repair Regen. 2006;14:162–71.
Li J, Zhao L, Yang T, Zeng YJ, Yang K. c-Ski inhibits autophagy of vascular smooth muscle cells induced by oxLDL and PDGF. PLoS One. 2014;9:e98902.
Sun D. The potential of endogenous neurogenesis for brain repair and regeneration following traumatic brain injury. Neural Regen Res. 2014;9:688–92.
Fernández-Hernández I, Rhiner C. New neurons for injured brains? The emergence of new genetic model organisms to study brain regeneration. Neurosci Biobehav Rev. 2015;56:62–72.
Zhang Y, Zhang ZG, Chopp M, Meng Y, Zhang L, Mahmood A, et al. Treatment of traumatic brain injury in rats with N-acetyl-seryl-aspartyl-lysyl-proline. J Neurosurg. 2017;126:782–95.
Wu H, Li J, Xu D, Zhang Q, Cui T. Growth differentiation factor 5 improves neurogenesis and functional recovery in adult mouse hippocampus following traumatic brain injury. Front Neurol. 2018;9:592.
Carlson SW, Saatman KE. Central infusion of insulin-like growth factor-1 increases hippocampal neurogenesis and improves neurobehavioral function after traumatic brain injury. J Neurotrauma. 2018;35:1467–80.
Zhou Y, Shao A, Yao Y, Tu S, Deng Y, Zhang J. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun Signal. 2020;18:62.
Mukherjee N, Nandi S, Garg S, Ghosh S, Ghosh S, Samat R, et al. Targeting chondroitin sulfate proteoglycans: an emerging therapeutic strategy to treat CNS injury. ACS Chem Neurosci. 2020;11:231–2.
Wanner I, Anderson M, Song B, Levine J, Fernandez A, Gray-Thompson Z, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–86.
Zhang Y, Chopp M, Meng Y, Zhang Z, Doppler E, Winter S, et al. Cerebrolysin improves cognitive performance in rats after mild traumatic brain injury. J Neurosurgery. 2015;122:843–55.
Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–27.
Zhang Y, Chopp M, Zhang ZG, Zhang Y, Zhang L, Lu M, et al. Cerebrolysin reduces astrogliosis and axonal injury and enhances neurogenesis in rats after closed head injury. Neurorehabil Neural Repair. 2019;33:15–26.
Xu W, Angelis K, Danielpour D, Haddad M, Bischof O, Campisi J, et al. Ski acts as a co-repressor with Smad2 and Smad3 to regulate the response to type beta transforming growth factor. Proc Natl Acad Sci USA. 2000;97:5924–9.
Reed J, Lin Q, Chen D, Mian I, Medrano E. SKI pathways inducing progression of human melanoma. Cancer Metastasis Rev. 2005;24:265–72.
Chen D, Xu W, Bales E, Colmenares C, Conacci-Sorrell M, Ishii S, et al. SKI activates Wnt/beta-catenin signaling in human melanoma. Cancer Res. 2003;63:6626–34.
Lambert C, Cisternas P, Inestrosa NC. Role of Wnt signaling in central nervous system injury. Mol Neurobiol. 2016;53:2297–311.
He Y, Zhang H, Yung A, Villeda SA, Jaeger PA, Olayiwola O, et al. ALK5-dependent TGF-beta signaling is a major determinant of late-stage adult neurogenesis. Nature Neurosci. 2014;17:943–52.
Dias JM, Alekseenko Z, Applequist JM, Ericson J. Tgfbeta signaling regulates temporal neurogenesis and potency of neural stem cells in the CNS. Neuron. 2014;84:927–39.
Zhang L, Yang X, Yang S, Zhang J. The Wnt /beta-catenin signaling pathway in the adult neurogenesis. Eur J Neurosci. 2011;33:1–8.
Liu R, Wang W, Wang S, Xie W, Li H, Ning B. microRNA-21 regulates astrocytic reaction post-acute phase of spinal cord injury through modulating TGF-β signaling. Aging (Albany NY). 2018;10:1474–88.
Yu Z, Yu P, Chen H, Geller HM. Targeted inhibition of KCa3.1 attenuates TGF-β-induced reactive astrogliosis through the Smad2/3 signaling pathway. J Neurochem. 2014;130:41–9.
Wang Y, Moges H, Bharucha Y, Symes A. Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp Neurol. 2007;203:168–84.
Susarla BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-beta-mediated induction of chondroitin sulfate proteoglycans. J Neurochem. 2011;119:868–78.
Minnich J, Mann S, Stock M, Stolzenbach K, Mortell B, Soderstrom K, et al. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects cortical neurons from dying following a traumatic brain injury. Restor Neurol Neurosci. 2010;28:293–309.
Hu J, Lang Y, Zhang T, Ni S, Lu H. Lentivirus-mediated PGC-1α overexpression protects against traumatic spinal cord injury in rats. Neuroscience. 2016;328:40–9.
Ren D, Zheng P, Feng J, Gong Y, Wang Y, Duan J, et al. Overexpression of astrocytes-specific GJA1-20k enhances the viability and recovery of the neurons in a rat model of traumatic brain injury. ACS Chem Neurosci. 2020;11:1643–50.
Xia C, Yin H, Yao Y, Borlongan C, Chao L, Chao J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther. 2006;17:206–19.
Yoo J, Seo J, Eom J, Hwang D. Effects of stromal cell-derived factor 1α delivered at different phases of transient focal ischemia in rats. Neuroscience. 2012;209:171–86.
Sugiura S, Kitagawa K, Tanaka S, Todo K, Omura-Matsuoka E, Sasaki T, et al. Adenovirus-mediated gene transfer of heparin-binding epidermal growth factor-like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke. 2005;36:859–64.
Yu Z, Chen L, Tang L, Hu C. Effects of recombinant adenovirus-mediated hypoxia-inducible factor-1alpha gene on proliferation and differentiation of endogenous neural stem cells in rats following intracerebral hemorrhage. Asian Pac J Trop Med. 2013;6:762–7.
Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus R, Young W, et al. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39:1254–61.
Shruster A, Ben-Zur T, Melamed E, Offen D. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS One. 2012;7:e40843.
Zeng L, He X, Liu J, Zheng C, Wang Y, Yang G. Lentivirus-mediated overexpression of microRNA-210 improves long-term outcomes after focal cerebral ischemia in mice. CNS Neurosci Ther. 2016;22:961–9.
Zhao Y, Wang L, Peng A, Liu X, Wang Y, Huang S, et al. The neuroprotective and neurorestorative effects of growth differentiation factor 11 in cerebral ischemic injury. Brain Res. 2020;1737:146802.
Guo Y, Liu S, Zhang X, Wang L, Zhang X, Hao A, et al. Sox11 promotes endogenous neurogenesis and locomotor recovery in mice spinal cord injury. Biochem Biophys Res Commun. 2014;446:830–5.
Chen Y, Ma N, Pei Z, Wu Z, Do-Monte F, Keefe S, et al. A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol Ther. 2020;28:217–34.
Zhang L, Lei Z, Guo Z, Pei Z, Chen Y, Zhang F, et al. Development of neuroregenerative gene therapy to reverse glial scar tissue back to neuron-enriched tissue. Front Cell Neurosci. 2020;14:594170.
Puls B, Ding Y, Zhang F, Pan M, Lei Z, Pei Z, et al. Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front Cell Dev Biol. 2020;8:591883.
Negro-Demontel M, Saccardo P, Giacomini C, Yáñez-Muñoz R, Ferrer-Miralles N, Vazquez E, et al. Comparative analysis of lentiviral vectors and modular protein nanovectors for traumatic brain injury gene therapy. Mol Ther Methods Clin Dev. 2014;1:14047.
Blanco-Ocampo D, Cawen F, Álamo-Pindado L, Negro-Demontel M, Peluffo H. Safe and neuroprotective vectors for long-term traumatic brain injury gene therapy. Gene Ther. 2020;27:96–103.
Acknowledgements
We thank Jin Bo and Dr Tong Hai-Peng from the Department of Radiology, Research Institute of Surgery and Daping Hospital for their technical assistance in MRI analysis. We thank the Department of Pathology, Research Institute of Surgery and Daping Hospital for their assistance in collecting and processing pathological specimens.
Funding
The research fund of the State Key Laboratory of Trauma, Burn and Combined Injury of China (No. SKLZZ20111) supported the purchase of reagents and experimental animals, the collection, analysis, and interpretation of data, and the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
YZhai and S-YY: carried out experiments, performed statistical analysis, and drafted the manuscript. YZhao, Y-LN, PL, and Y-GZ: designed and oversaw the experiments, interpreted the data, and reviewed the manuscript. HD, Z-ZH: carried out a significant proportion of the supplementary experiments. Q-SW: performed the preparation of paraffin sections, and analyses of neuropathological characterization. R-PX: performed the western blot experiments and corresponding statistical analysis. Y-WX: assisted in the neurobehavioral examination. S-YF, YP, and NY: assisted in making animal models, and the injection of Adv. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhai, Y., Ye, SY., Wang, QS. et al. Overexpressed ski efficiently promotes neurorestoration, increases neuronal regeneration, and reduces astrogliosis after traumatic brain injury. Gene Ther 30, 75–87 (2023). https://doi.org/10.1038/s41434-022-00320-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-022-00320-x