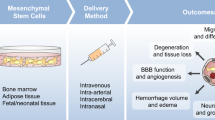
Abstract
Intracerebral hemorrhage (ICH) is a life-threatening condition with a high mortality rate. For survivors, quality of life is determined by primary and secondary phases of injury. The prospects for injury repair and recovery after ICH are highly dependent on the extent of secondary injury. Currently, no effective treatments are available to prevent secondary injury or its long-term effects. One promising strategy that has recently garnered attention is gene therapy, in particular, small interfering RNAs (siRNA), which silence specific genes responsible for destructive effects after hemorrhage. Gene therapy as a potential treatment for ICH is being actively researched in animal studies. However, there are many barriers to the systemic delivery of siRNA-based therapy, as the use of naked siRNA has limitations. Recently, the Food and Drug Administration approved two siRNA-based therapies, and several are undergoing Phase 3 clinical trials. In this review, we describe the advancements in siRNA-based gene therapy for ICH and also summarize its advantages and disadvantages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–31.
Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–69.
Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nature Reviews. Neurology. 2010;6:593–601.
Shao Z, Tu S, Shao A. Pathophysiological mechanisms and potential therapeutic targets in intracerebral hemorrhage. Front Pharmacol. 2019;10:1079.
Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–99.
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. New Engl J Med. 2001;344:1450–60.
Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–51.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet (London, England). 2009;373:1632–44.
Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–9.
Lusardi TA, Wolf JA, Putt ME, Smith DH, Meaney DF. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J Neurotrauma. 2004;21:61–72.
Lan X, Han X, Li Q, Li Q, Gao Y, Cheng T, et al. Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia. Brain, Behav Immun. 2017;61:326–39.
Tang R, Huang Z, Chu H. Phenotype Change of Polarized Microglia after Intracerebral Hemorrhage: Advances in Research. Brain Hemorrhages. 20201:161–5.
Xiong X-Y, Liu L, Wang F-X, Yang Y-R, Hao J-W, Wang P-F, et al. Toll-like receptor 4/MyD88–mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation. 2016;134:1025–38.
Fang H, Wang P-F, Zhou Y, Wang Y-C, Yang Q-W. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflamm. 2013;10:1–10.
Rodríguez-Yáñez M, Brea D, Arias S, Blanco M, Pumar JM, Castillo J, et al. Increased expression of Toll-like receptors 2 and 4 is associated with poor outcome in intracerebral hemorrhage. J Neuroimmunol. 2012;247:75–80.
Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42:1781–6.
Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–77.
Hwang BY, Appelboom G, Ayer A, Kellner CP, Kotchetkov IS, Gigante PR, et al. Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovasc Dis (Basel, Switzerland). 2011;31:211–22.
Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908.
Lan X, Han X, Li Q, Yang Q-W, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nature Reviews. Neurology. 2017;13:420–33.
Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Dávalos A, et al. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36:86–91.
Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2:e90777.
Mohammed Thangameeran SI, Tsai S-T, Hung H-Y, Hu W-F, Pang C-Y, Chen S-Y, et al. A role for endoplasmic reticulum stress in intracerebral hemorrhage. Cells. 2020;9:750.
Alhadidi Q, Shah ZA. Cofilin mediates LPS-induced microglial cell activation and associated neurotoxicity through activation of NF-κB and JAK–STAT pathway. Mol Neurobiol. 2018;55:1676–91.
Alhadidi Q, Nash KM, Alaqel S, Sayeed MSB, Shah ZA. Cofilin knockdown attenuates hemorrhagic brain injury-induced oxidative stress and microglial activation in mice. Neuroscience. 2018;383:33–45.
Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272–8.
Yang Y, Zhang Y, Wang Z, Wang S, Gao M, Xu R, et al. Attenuation of acute phase injury in rat intracranial hemorrhage by cerebrolysin that inhibits brain edema and inflammatory response. Neurochem Res. 2016;41:748–57.
Li N, Worthmann H, Heeren M, Schuppner R, Deb M, Tryc AB, et al. Temporal pattern of cytotoxic edema in the perihematomal region after intracerebral hemorrhage: a serial magnetic resonance imaging study. Stroke. 2013;44:1144–6.
Wu H, Wu T, Han X, Wan J, Jiang C, Chen W, et al. Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. J Cereb Blood Flow Metab. 2017;37:39–51.
Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13:420–33.
Madangarli N, Bonsack F, Dasari R, Sukumari-Ramesh S. Intracerebral Hemorrhage: Blood Components and Neurotoxicity. Brain sciences. 2019;9.
Fang M, Zhong L, Jin X, Cui R, Yang W, Gao S, et al. Effect of Inflammation on the Process of Stroke Rehabilitation and Poststroke Depression. Front Psychiatry. 2019;10:184.
Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:1–25.
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178:101610.
Lok J, Zhao S, Leung W, Seo JH, Navaratna D, Wang X, et al. Neuregulin-1 effects on endothelial and blood–brain barrier permeability after experimental injury. Translational Stroke Res. 2012;3:119–24.
Fukuda AM, Badaut J. siRNA treatment: “A sword-in-the-Stone” for acute brain injuries. Genes. 2013;4:435–56.
Zhou K, Shi L, Wang Y, Chen S, Zhang J. Recent advances of the NLRP3 inflammasome in central nervous system disorders. Journal of Immunology Research. 2016;2016.
Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187:597–602.
Xiao L, Zheng H, Li J, Wang Q, Sun H. Neuroinflammation mediated by NLRP3 inflammasome after intracerebral hemorrhage and potential therapeutic targets. Mol Neurobiol. 2020;57:5130–49.
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–19.
Feng L, Chen Y, Ding R, Fu Z, Yang S, Deng X, et al. P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: involvement of peroxynitrite. J Neuroinflamm. 2015;12:1–17.
Wang L, Zheng S, Zhang L, Xiao H, Gan H, Chen H, et al. HDAC10 alleviates inflammation after intracerebral hemorrhage via the PTPN22/NLRP3 pathway in rats. Neuroscience. 2020;432:247–59.
Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2011;31:881–93.
Zhang J, Dong B, Hao J, Yi S, Cai W, Luo Z. LncRNA Snhg3 contributes to dysfunction of cerebral microvascular cells in intracerebral hemorrhage rats by activating the TWEAK/Fn14/STAT3 pathway. Life Sci. 2019;237:116929.
Li Z, He Q, Zhai X, You Y, Li L, Hou Y, et al. Foxo1-mediated inflammatory response after cerebral hemorrhage in rats. Neurosci Lett. 2016;629:131–6.
Yang P, Wu J, Miao L, Manaenko A, Matei N, Zhang Y, et al. Platelet-derived growth factor receptor-β regulates vascular smooth muscle cell phenotypic transformation and neuro-inflammation after intracerebral hemorrhage in mice. Criti Care Med. 2016;44:e390–402.
Wu J, Sun L, Li H, Shen H, Zhai W, Yu Z, et al. Roles of programmed death protein 1/programmed death-ligand 1 in secondary brain injury after intracerebral hemorrhage in rats: selective modulation of microglia polarization to anti-inflammatory phenotype. J Neuroinflamm. 2017;14:36.
Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69.
Jin X, Ishii H, Bai Z, Itokazu T, Yamashita T. Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One. 2012;7:e41892.
Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, et al. Microglia activation and polarization after intracerebral hemorrhage in mice: the role of protease-activated receptor-1. Translational Stroke Res. 2016;7:478–87.
Zhao H, Garton T, Keep RF, Hua Y, Xi G. Microglia/Macrophage Polarization After Experimental Intracerebral Hemorrhage. Translational Stroke Res. 2015;6:407–9.
Chen ZQ, Yu H, Li HY, Shen HT, Li X, Zhang JY, et al. Negative regulation of glial Tim‐3 inhibits the secretion of inflammatory factors and modulates microglia to antiinflammatory phenotype after experimental intracerebral hemorrhage in rats. CNS Neurosci Therapeutics. 2019;25:674–84.
Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, et al. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22:404–10.
Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:5612.
Agnihotri S, Wolf A, Munoz DM, Smith CJ, Gajadhar A, Restrepo A, et al. A GATA4-regulated tumor suppressor network represses formation of malignant human astrocytomas. J Exp Med. 2011;208:689–702.
Xu H, Cao J, Xu J, Li H, Shen H, Li X, et al. GATA-4 regulates neuronal apoptosis after intracerebral hemorrhage via the NF-κB/Bax/Caspase-3 pathway both in vivo and in vitro. Exp Neurol. 2019;315:21–31.
Zhang R, Liu Y, Yan K, Chen L, Chen X-R, Li P, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflamm. 2013;10:1–12.
Shalaby SM, El-Shal AS, Abd-Allah SH, Selim AO, Selim SA, Gouda ZA, et al. Mesenchymal stromal cell injection protects against oxidative stress in Escherichia coli–induced acute lung injury in mice. Cytotherapy. 2014;16:764–75.
Bedini G, Bersano A, Zanier ER, Pischiutta F, Parati EA. Mesenchymal stem cell therapy in intracerebral haemorrhagic stroke. Curr Medicinal Chem. 2018;25:2176–97.
Chen X, Liang H, Xi Z, Yang Y, Shan H, Wang B, et al. BM-MSC Transplantation Alleviates Intracerebral Hemorrhage-Induced Brain Injury, Promotes Astrocytes Vimentin Expression, and Enhances Astrocytes Antioxidation via the Cx43/Nrf2/HO-1 Axis. Front Cell Developmental Biol. 2020;8:302.
Lei C, Lin S, Zhang C, Tao W, Dong W, Hao Z, et al. Activation of cerebral recovery by matrix metalloproteinase-9 after intracerebral hemorrhage. Neuroscience. 2013;230:86–93.
Campbell M, Hanrahan F, Gobbo OL, Kelly ME, Kiang A-S, Humphries MM, et al. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat Commun. 2012;3:1–12.
Weng Y, Xiao H, Zhang J, Liang X-J, Huang Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol Adv. 2019;37:801–25.
Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angewandte Chemie. 2012;124:8657–61.
Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:1–9.
Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Nat Acad Sci. 2007;104:12982–7.
Brown BD, Wang W. Ligand-Modified Double-Stranded Nucleic Acids. Google Patents; 2017.
Thapar M, Rudnick S, Bonkovsky HL. Givosiran, a novel treatment for acute hepatic porphyrias. Exp Rev Precision Med Drug Develop. 2021;6:9–18.
Yuan-Yu H. Approval of the first-ever RNAi therapeutics and its technological development history. Prog Biochem Biophys. 2019;46:313–22.
Li Z, You M, Long C, Bi R, Xu H, He Q, et al. Hematoma expansion in intracerebral hemorrhage: an update on prediction and treatment. Front Neurol. 2020;11:702.
Tam C, Wong JH, Cheung RCF, Zuo T, Ng TB. Therapeutic potentials of short interfering RNAs. Appl Microbiol Biotechnol. 2017;101:7091–111.
Tai W. Current aspects of siRNA bioconjugate for in vitro and in vivo delivery. Molecules. 2019;24:2211.
Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Controlled Release. 2016;235:34–47.
Wang Y, Li P, Kong L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. Aaps Pharmscitech. 2013;14:585–92.
Tosi G, Costantino L, Ruozi B, Forni F, Vandelli MA. Polymeric nanoparticles for the drug delivery to the central nervous system. Expert Opin Drug Delivery. 2008;5:155–74.
Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–28.
Wang SS, Chou NK, Chung TW. The t‐PA‐encapsulated PLGA nanoparticles shelled with CS or CS‐GRGD alter both permeation through and dissolving patterns of blood clots compared with t‐PA solution: An in vitro thrombolysis study. J Biomed Mat Res Part A. 2009;91:753–61.
Funding
Daniyah Almarghalani was supported by a scholarship from Taif University, Saudi Arabia Cultural Mission. The study was partly supported by grants from the American Heart Association #17AIREA33700076/ZAS/2017 and National Institute of Neurological Disorders and Stroke of the National Institutes of Health #R01NS112642 to ZAS.
Author information
Authors and Affiliations
Contributions
DAA contributed to performing the literature search, designing the review structure, and writing the first draft. ZAS contributed to reviewing, providing feedback, and approving the final draft.
Corresponding author
Ethics declarations
COMPETING INTERESTS
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almarghalani, D.A., Shah, Z.A. Progress on siRNA-based gene therapy targeting secondary injury after intracerebral hemorrhage. Gene Ther 30, 1–7 (2023). https://doi.org/10.1038/s41434-021-00304-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00304-3
This article is cited by
-
Blood-spinal cord barrier disruption in degenerative cervical myelopathy
Fluids and Barriers of the CNS (2023)