Abstract
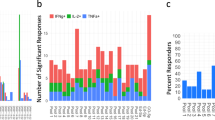
Immune responses to Cas proteins have been demonstrated recently and these may prove to be an impediment to their clinical use in gene editing. To make meaningful assessments of Cas9 immunogenicity during drug development and licensure it is imperative the reagents are free of impurities that could affect in vitro assessments of immunogenicity. Here we address the issue of endotoxin levels in laboratory grade Cas9 proteins used to measure T-cell memory responses. Many of these reagents have not been developed for immunogenicity assays, are or microbial origin and carry varying levels of endotoxin. The use of these reagents, off the shelf, without measuring endotoxin levels is likely to introduce incorrect estimates of the prevalence of memory T-cell responses in research studies. We demonstrate wide variation in endotoxin levels in Cas9 proteins from seven suppliers. Different lots from the same supplier also contained varying levels of endotoxin. ELISPOT assays showed similar large variations in the interferon-γ signals. Finally, when we carried out endotoxin depletion in four Cas9 proteins with strong signals in the ELISPOT assay, we found dampening of the interferon-γ signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gaj T, Sirk SJ, Shui SL, Liu J. Genome-editing technologies: principles and applications. Cold Spring Harb Perspect Biol. 2016;8:a023754.
Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5:1.
Pickar-Oliver A, Gersbach CA. The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol. 2019;20:490–507.
Rodriguez-Rodriguez DR, Ramirez-Solis R, Garza-Elizondo MA, Garza-Rodriguez ML, Barrera-Saldana HA. Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases (Review). Int J Mol Med. 2019;43:1559–74.
Chen H, Shi M, Gilam A, Zheng Q, Zhang Y, Afrikanova I, et al. Hemophilia A ameliorated in mice by CRISPR-based in vivo genome editing of human Factor VIII. Sci Rep. 2019;9:16838.
Chew WL, Tabebordbar M, Cheng JK, Mali P, Wu EY, Ng AH, et al. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat Methods. 2016;13:868–74.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91.
Stone D, Long KR, Loprieno MA, De Silva Feelixge HS, Kenkel EJ, Liley RM, et al. CRISPR-Cas9 gene editing of hepatitis B virus in chronically infected humanized mice. Mol Ther Methods Clin Dev. 2021;20:258–75.
Charlesworth CT, Deshpande PS, Dever DP, Camarena J, Lemgart VT, Cromer MK, et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25:249–54.
Simhadri VL, McGill J, McMahon S, Wang J, Jiang H, Sauna ZE. Prevalence of Pre-existing Antibodies to CRISPR-Associated Nuclease Cas9 in the USA Population. Mol Ther Methods Clin Dev. 2018;10:105–12.
Wagner DL, Amini L, Wendering DJ, Burkhardt LM, Akyuz L, Reinke P, et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat Med. 2019;25:242–8.
Food and Drug Administration. Immunogenicity Assessment for Therapeutic Protein Products (Guidance for Industry) 2014.
Rosenberg AS, Sauna ZE. Immunogenicity assessment during the development of protein therapeutics. J Pharm Pharmacol. 2018;70:584–94.
Fessenden M. Technologies to watch in 2019. Nature. 2019;565:521–3.
Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202.
Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–4.
Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34.
Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446.
Schwarz H, Gornicec J, Neuper T, Parigiani MA, Wallner M, Duschl A, et al. Biological Activity of Masked Endotoxin. Sci Rep. 2017;7:44750.
Ferdosi SR, Ewaisha R, Moghadam F, Krishna S, Park JG, Ebrahimkhani MR, et al. Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat Commun. 2019;10:1842.
Rosenberg AS, Worobec, A. A Risk-Based Approach to Immunogenicity Concerns of Therapeutic Protein Products, Part 2: Considering Host-Specific and Product-Specific Factors Impacting Immunogenicity. BioPharm International. 2004;17:12.
Budwell G. Uh-Oh! CRISPR Gene-Editing Stocks May Be Worthless. 2018.
Daley J. Immunity May Make CRISPR-Based Therapies Ineffective. TheScientist. 2018.
Ledford H. How the immune system could stymie some CRISPR gene therapies. Nature. 2018.
Zhang S. You May Already Be Immune to CRISPR. 2018.
Tennenberg SD, Weller JJ. Endotoxin activates T cell interferon-gamma secretion in the presence of endothelium. J Surg Res. 1996;63:73–6.
Dawson M. ENDOTOXIN LIMITS for Parenteral Drug Products. BET White Paper. 2017;1:2.
Food and Drug Administration. Pyrogen and Endotoxins Testing: Questions and Answers (Guidance for Industry). 2012.
The United States Pharmacopeial Convention. Bacterial Endotoxin Test. Second Supplement to USP 35–NF 30. 2012.
Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101.
Rock KL, Reits E, Neefjes J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016;37:724–37.
Weenink SM, Gautam AM. Antigen presentation by MHC class II molecules. Immunol Cell Biol. 1997;75:69–81.
La Gruta NL, Gras S, Daley SR, Thomas PG, Rossjohn J. Understanding the drivers of MHC restriction of T cell receptors. Nat Rev Immunol. 2018;18:467–78.
Ratanji KD, Dearman RJ, Kimber I, Thorpe R, Wadhwa M, Derrick JP. Editor’s Highlight: Subvisible Aggregates of Immunogenic Proteins Promote a Th1-Type Response. Toxicol Sci. 2016;153:258–70.
Endotoxin-free protein production-ClearColiTM technology. Nature Methods. 2013;10:916.
Varma TK, Lin CY, Toliver-Kinsky TE, Sherwood ER. Endotoxin-induced gamma interferon production: contributing cell types and key regulatory factors. Clin Diagn Lab Immunol. 2002;9:530–43.
Alagoz M, Kherad N. Advance genome editing technologies in the treatment of human diseases: CRISPR therapy (Review). Int J Mol Med. 2020;46:521–34.
Cai L, Fisher AL, Huang H, Xie Z. CRISPR-mediated genome editing and human diseases. Genes Dis. 2016;3:244–51.
Acknowledgements
ZES is funded by intramural grants from the US Food and Drug Administration. Custom manufactured, endotoxin free products, CON-SaCas9 and CON-SpCas9 were a kind gift from Editas Medicine.
Author information
Authors and Affiliations
Contributions
ZES, conceptualised the study. VLS, performed experiments. ZES, VLS, JRM, designed experiments and analysed data. ZES, VLS, JRM, wrote the paper. ZES obtained funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Simhadri, V.L., McGill, J.R. & Sauna, Z.E. Endotoxin contamination in commercially available Cas9 proteins potentially induces T-cell mediated responses. Gene Ther 30, 575–580 (2023). https://doi.org/10.1038/s41434-021-00301-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00301-6