Abstract
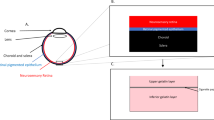
Retinal damage has been associated with increased injection pressure during subretinal gene therapy delivery in various animal models, yet there are no human clinical data regarding the pressures required to initiate and propagate subretinal blebs. This study characterized the intraoperative pressure levels for subretinal gene therapy delivery across eight retinal conditions. A total of 116 patients with retinal degenerative diseases have been treated with subretinal gene therapy at OHSU-Casey Eye Institute as of June 2020; seventy patients (60.3%) were treated using a pneumatic-assisted subretinal delivery system. All retinal blebs were performed using a 41-gauge injection cannula, and use of a balanced salt solution (BSS) “pre-bleb” prior to gene therapy delivery was performed at the discretion of the surgeon. Patient age and intraoperative data for BSS and vector injections were analyzed in a masked fashion for all patients who received pneumatic-assisted subretinal gene therapy. The median age of the patients was 35 years (range 4–70). No significant differences in injection pressures were found across the eight retinal conditions. In this study, patient age was shown to affect maximum injection pressures required for bleb propagation, and the relationship between age and pressure varied based on retinal condition. These data have important implications in optimizing surgical protocols for subretinal injections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated or analysed during this study can be found within the published article and its supplementary files.
References
Russell S, Bennett J, Wellman J, Chung D, Yu Z, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60. https://doi.org/10.1016/S0140-6736(17)31868-8
Cehajic-Kapetanovic J, Xue K, Martinez-Fernandez de la Camara C, Nanda A, Davies A, Wood L, et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020;26:354–9. https://doi.org/10.1038/s41591-020-0763-1
Fischer M, Ochakovski G, Beier B, Seitz I, Vaheb Y, Kortuem C, et al. Efficacy and safety of retinal gene therapy using adeno-associated virus vector for patients with choroideremia: a randomized clinical trial. JAMA Ophthalmol. 2019;137:1247–54. https://doi.org/10.1001/jamaophthalmol.2019.3278
Cukras C, Wiley H, Jeffrey B, Sen H, Turriff A, Zeng Y, et al. Retinal AAV8-RS1 gene therapy for X-Linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. 2018;26:2282–94. https://doi.org/10.1016/j.ymthe.2018.05.025
MacLaren R, Groppe M, Barnard A, Cottriall C, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37. https://doi.org/10.1016/S0140-6736(13)62117-0
Davis J, Gregori N, MacLaren R, Lam B. Surgical technique for subretinal gene therapy in humans with inherited retinal degeneration. Retina. 2019;39:S2–S8. https://doi.org/10.1097/IAE.0000000000002609. Suppl 1
Scruggs B, Jiao C, Cranston C, Kaalberg E, Wang K, Russell S, et al. Optimizing donor cellular dissociation and subretinal injection parameters for stem cell-based treatments. Stem Cells Transl Med. 2019;8:797–809. https://doi.org/10.1002/sctm.18-0210
Takahashi K, Morizane Y, Hisatomi T, Tachibana T, Kimura S, Hosokawa M, et al. The influence of subretinal injection pressure on the microstructure of the monkey retina. PLoS One. 2018;13:e0209996. https://doi.org/10.1371/journal.pone.0209996
Xue K, Groppe M, Salvetti A, MacLaren R. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye. 2017;31:1308–16. https://doi.org/10.1038/eye.2017.158
Peng Y, Tang L, Zhou Y. Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58:217–26. https://doi.org/10.1159/000479157
Vasconcelos H, Lujan B, Pennesi M, Yang P, Lauer A. Intraoperative optical coherence tomographic findings in patients undergoing subretinal gene therapy surgery. Int J Retina Vitreous. 2020;6:13. https://doi.org/10.1186/s40942-020-00216-1
Gregori N, Lam B, Davis J. Intraoperative use of microscope-integrated optical coherence tomography for subretinal gene therapy delivery. Retina. 2019;39 Suppl 1:S9–S12. https://doi.org/10.1097/IAE.0000000000001646
Gange W, Sisk R, Besirli C, Lee T, Havunjian M, Schwartz H, et al. Perifoveal chorioretinal atrophy following subretinal voretigene neparvovec-rzyl for RPE65-mediated Leber Congenital Amaurosis. Ophthalmol Retina. 2021;S2468-6530 00106-8. https://doi.org/10.1016/j.oret.2021.03.016
Acknowledgements
We acknowledge the support of NIH P30EY010572, The Heed Foundation, and an unrestricted grant to the Casey Eye Institute Department of Ophthalmology from Research to Prevent Blindness, Inc. New York, NY. We would like to thank Sheila Markwardt, MPH for her expertise and assistance in statistical analysis.
Funding
We acknowledge the support of NIH P30EY010572, NIH K08EY026650 (PY), Foundation Fighting Blindness Career Development Award CD-NMT-0714-0648 (PY), The Heed Foundation (BAS), and an unrestricted grant to the Casey Eye Institute Department of Ophthalmology from Research to Prevent Blindness, Inc. New York, NY.
Author information
Authors and Affiliations
Contributions
BAS was responsible for the conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing. HMV was responsible for the conception and design, collection and/or assembly of data, and data analysis and interpretation. MMDP was responsible for collection and/or assembly of data and data analysis and interpretation. KK was responsible for collection and/or assembly of data and data analysis and interpretation. MEP was responsible for data analysis and interpretation and manuscript writing. PY was responsible for data analysis and interpretation and manuscript writing. STB was responsible for collection and/or assembly of data, data analysis and interpretation, and manuscript writing. AKL was responsible for the conception and design, collection and/or assembly of data, financial support, administrative support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
AKL has received consulting fees or funds in support of clinical research from AGTC, Allergan, Atsena, Biogen, Cambridge Consulting, Genentech, IvericBio, Oxford BioMedic, Regenxbio, TeamedOn. MKP has received consulting fees from Adverum, AGTC, Allergan, Astellas Pharmaceuticals, Biogen, BlueRock, Editas, Iveric Bio, Novartis, Ora, RegenexBio, Roche, Viewpoint Therapeutics. MKP serves on the scientific advisor boards for Atsena, DTx Therapeutics, Endogena, Eyevensys, Horama, Nayan, Nacuity Pharmaceuticals, Ocugen, Sparing Vision, and Vedere. PY has received consulting fees from Adverum, AGTC, Nanoscope Therapeutics. All other authors declare no potential competing interests.
Ethical approval
Institutional Review Board approval at OHSU was obtained for review of all operative notes of patients receiving subretinal delivery of gene therapy.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Scruggs, B.A., Vasconcelos, H.M., Matioli da Palma, M. et al. Injection pressure levels for creating blebs during subretinal gene therapy. Gene Ther 29, 601–607 (2022). https://doi.org/10.1038/s41434-021-00294-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00294-2