Abstract
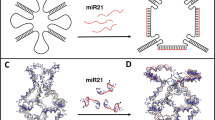
Nowadays, nano-compartments are considered as an effective drug delivery system (DDS) for cancer therapy. Targeted delivery of therapeutic agents is an advantageous approach by which cancer cells can be targeted without harming normal cells, and eliminates the negative effects of conventional therapies such as chemotherapy. In this research, a novel zinc-based nanoscale metal-organic framework (Zn-NMOF) coated with folic acid (FA) functionalized chitosan (CS) has been constructed and applied as efficient delivery of LNA (locked nucleic acid)-antisense miR-224 to colon cancer cell lines. LNA-antisense miR-224 as a therapeutic sequence was able to considerably block highly expressed miR-224 and downregulated cancer cell growth. The prepared nano-complex was characterized by analytical devices such as FT-IR, UV-Vis spectrophotometry, DLS, TEM, and XRD. The size range of NMOF-CS-FA-LNA-antisense miR-224 (MCFL224) nano-complex was obtained nearly at 200 nm. The entrapment efficiency of LNA-antisense miR-224 was calculated 72 ± 5% and a significant release profile of LNA-antisense miR-224 was observed at first 6 h (about 50%). Then, in vitro assays were implemented on HCT116 (folic acid receptor-positive colon cancer cell line) and CRL1831 (normal colon cell line) to evaluate the therapeutic efficiency of the MCFL224 nano-complex. In these investigations, decreased cell viability (14.22 ± 0.3% after 72 h treatment), increased apoptotic and autophagy-related genes expression level (BECLIN1: 34-folds, BAX: 36-folds, mTORC1: 10-folds, and Caspase-9: 9-folds more than control), higher cell cycle arrest in sub-G1 phase (19.53% of cells in sub-G1 phase), and more apoptosis analyses (late apoptosis: 67.7%) were evaluated in colon cancer cells treated with MCFL224 nano-complex. Results remarkably indicate the inhibited growth of colon cancer cells and induced cell apoptosis which suggests MCFL224 as a promising nanocomposite for colon cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Narmani A, Kamali M, Panahi Y, Amini B, Salimi A. Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: in vitro studies. Process Biochem. 2018;69:178–87.
Ding S, Khan AI, Cai X, Song Z, Lyu Z, Du D, et al. Overcoming blood-brain barrier transport: advances in nanoparticle-based drug delivery strategies. Mater Today. https://doi.org/10.1016/j.mattod.2020.02.001.
Narmani A, Farhood B, Haghi-Aminjan H, Mortezazadeh T, Aliasgharzadeh A, Mohseni M, et al. Gadolinium nanoparticles as diagnostic and therapeutic agents: their delivery systems in magnetic resonance imaging and neutron capture therapy. J Drug Deliv Sci Technol. 2018;44:457–66.
Kumari R, Sunil D, Ningthoujam RS. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review. J Control Release. 2020;319:135–56.
Yousefi M, Narmani A, Jafari SM. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv. Colloid Interface Sci. 2020;278:102125.
Wu D, Zhu L, Li Y, Zhang X, Xu S, Yang G, et al. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: a review. Carbohydr Polym. 2020;238:116126.
Narmani A, Kamali M, Amini B, Kooshki H, Amini A, Hassani L. Highly sensitive and accurate detection of Vibrio cholera O1 OmpW gene by fluorescence DNA biosensor based on gold and magnetic nanoparticles. Process Biochem. 2018;65:46–54.
Wang XG, Dong ZY, Cheng H, Wan SS, Chen WH, Zou MZ, et al. A multifunctional metal–organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale. 2015;7:16061–70.
Zhuang J, Kuo CH, Chou LY, Liu DY, Weerapana E, Tsung CK. Optimized metal_organic- framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8:2812–9.
Liang K, Wang R, Boutter M, Doherty CM, Mulet X, Richardson JJ. Biomimetic mineralization of metal–organic frameworks around polysaccharides. Chem Commun. 2017;19:1249–52.
Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nystrom AM, et al. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138:962–8.
Narmani A, Rezvani M, Farhood B, Darkhor P, Mohammadnejad J, Amini B, et al. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res. 2019;80:404–24.
Narmani A, Mohammadnejad J, Yavari K. Synthesis and evaluation of polyethylene glycol- and folic acid-conjugated polyamidoamine G4 dendrimer as nanocarrier. J Drug Deliv Sci Technol. 2019;50:278–86.
Narmani A, Arani MAA, Mohammadnejad J, Vaziri AZ, Solymani S, Yavari K, et al. Breast tumor targeting with PAMAM-PEG-5FU-99mTc as a new therapeutic nanocomplex: in in-vitro and in-vivo studies. Biomed Microdevices. 2020;22:31.
Chen Y, Wu D, Zhong W, Kuang S, Luo Q, Song L, et al. Evaluation of the PEG fensity in the PEGylated chitosan nanoparticles as a drug carrier for curcumin and mitoxantrone. Nanomaterials. 2018;8:486.
Rezvani M, Mohammadnejad J, Narmani A, Bidaki K. Synthesis and in vitro study of modified chitosan polycaprolactamnanocomplex as delivery system. Int J Biol Macromol. 2018;113:1287–93.
Mombini S, Mohammadnejad J, Bakhshandeh B, Narmani A, Nourmohammadi J, Vahdat S, et al. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int J Biol Macromol. 2019;140:278–87.
Duan J, Liang X, Cao Y, Wang S, Zhang L. pH-sensitive polymeric micelles for targeted delivery to inflamed joints. Macromolecules. 2015;48:2706–14.
Jiang YW, Chen LA. microRNAs as tumor inhibitors, oncogenes, biomarkers for drug efficacy and outcome predictors in lung cancer (review). Mol Med Rep. 2012;5:890–4.
Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–84.
Liao W-T, Li T-T, Wang Z-G, Wang S-Y, He M-R, Ye Y-P, et al. microRNA-224 promotes cell proliferation and tumor growth in human colorectal cancer by repressing PHLPP1 and PHLPP2. Clin Cancer Res. 2013;19:4662.
Huang L, Dai T, Lin X, Zhao X, Chen X, Wang C, et al. MicroRNA-224 targets RKIP to control cell invasion and expression of metastasis genes in human breast cancer cells. Biochem Biophys Res Commun. 2012;425:127–33.
Hidalgo T, Giménez-Marqués M, Bellido E, Avila J, Asensio MC, Salles F, et al. Chitosan-coated mesoporous MIL-100 (Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci Rep. 2017;7:43099.
Daryasari MP, Akhgar MR, Mamashli F, Bigdeli B, Khoobi M. Carbon dots embedded magnetic nanoparticles@ chitosan@ metal organic framework as a nanoprobe for pH sensitive targeted anticancer drug delivery. RSC Adv. 2016;107:105578–88.
Narmani A, Yavari K, Mohammadnejad J. Imaging, biodistribution and in vitro study of smart 99mTc-PAMAM G4 dendrimer as novel nano-complex. Colloids Surf B. 2017;159:232–40.
SPECIFICATIONS Q, DOCUMENTATION P. Lipofectamine™ 2000 Transfection Reagent.
Bwatanglang IB, Mohammad F, Yusof NA, Abdullah J, Alitheen NB, Hussein MZ, et al. In vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. J Colloid Interface Sci. 2016;480:146–58.
Ghaffar I, Imran M, Perveen S, Kanwal T, Saifullah S, Bertino MF. Synthesis of chitosan coated metal organic frameworks (MOFs) for increasing vancomycin bactericidal potentials against resistant S. aureus strain. Mater Sci Eng C. 2019;105:110111.
Liu L, Ping E, Sun J, Zhang L, Zhou Y, Zhong Y, et al. Multifunctional Ag@ MOF-5@chitosan non-woven cloth composites for sulfur mustard decontamination and hemostasis. Dalton Trans. 2019;48:6951–9.
Yang L, Ruess GL, Carreon MA. Cu, Al and Ga based metal organic framework catalysts for the decarboxylation of oleic acid. Catal Sci Technol. 2015;5:2777–82.
Ali MEA, Aboelfadl MMS, Selim AM, Khalil HF, Elkady GM. Chitosan nanoparticles extracted from shrimp shells, application for removal of Fe(II) and Mn(II) from aqueous phases. Sep Sci Technol. 2018;53:2870–81.
Chalati T, Horcajada P, Gref R, Couvreur P, Serre C. Optimisation of the synthesis of MOF nanoparticles made of flexible porous iron fumarate MIL-88A. J Mater Chem. 2011;21:2220–7.
Abazari R, Mahjoub AR, Ataei F, Morsali A, Carpenter-Warren CL, Mehdizadeh K, et al. Chitosan immobilization on bio-MOF nanostructures: a biocompatible pH-responsive nanocarrier for doxorubicin release on MCF-7 cell lines of human breast cancer. Inorg Chem. 2018;57:13364–79.
Hajiashrafi S, Kazemi NM. Preparation and evaluation of ZnO nanoparticles by thermal decomposition of MOF-5. Heliyon. 2019;5:e02152.
Cosco D, Cilurzo F, Maiuolo J, Federico C, Martino MTD, Cristiano MC. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci Rep. 2015;5:17579.
Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35:4333–44.
Tekie FSM, Atyabi F, Soleimani M, Arefian E, Atashi A, Kiani M, et al. Chitosan polyplex nanoparticle vector for miR-145 expression in MCF-7: optimization by the design of experiment. Int J Biol Macromol. 2015;81:828–37.
Ju E, Li T, Liu Z, da Silva SR, Wei S, Zhang X, et al. Specific inhibition of viral microRNAs by carbon dots-mediated delivery of locked nucleic acids for therapy of virus-induced cancer. ACS Nano. 2020;14:476–87.
Lin Q, Mao Y, Song Y, Huang D. MicroRNA-34a induces apoptosis in PC12 cells by reducing B-cell lymphoma 2 and sirtuin-1 expression. Mol Med Rep. 2015;12:5709–14.
Wu J, Wu G, Lv L, Ren YF, Zhang XJ, Xue YF, et al. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis. 2012;33:519–28.
Zou M, Lu N, Hu C, Liu W, Sun Y, Wang X, et al. Beclin 1-mediated autophagy in hepatocellular carcinoma cells: Implication in anticancer efficiency of oroxylin A via inhibition of mTOR signaling. Cell Signal. 2012;24:1722–32.
Towers CG, Wodetzki D, Thorburn A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations. Journal of Cell Biology. 2020;219:e201907022.
Levy JM, Thorburn A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27:843–57.
Murad H, Hawat M, Ekhtiar A, AlJapawe A, Abbas A, Darwish H, et al. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016;16:1–1.
Karsy M, Arslan E, Moy F. Current progress on understanding microRNAs in glioblastoma multiforme. Genes Cancer. 2012;3:3–15.
Tu L, Wang M, Zhao W-Y, Zhang Z-Z, Tang D-F, Zhang Y-Q. miRNA-218-loaded carboxymethyl chitosan-tocopherol nanoparticle to suppress the proliferation of gastrointestinal stromal tumor growth. Mater Sci Eng C. 2017;72:177–84.
Wang L, Zhou B–B, Yu K, Su Z–H, Gao S, Chu L–L, et al. Novel antitumor agent, Trilacunary Keggin–type tungstobismuthate, inhibits proliferation and induces apoptosis in human gastric cancer SGC–7901 cells. Inorg. Chem. 2013;52:5119–27.
Howe EN, Cochrane DR, Richer JK. Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res. 2011;13:R45.
Stein CA, Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol Ther. 2017;25:1069–75.
Krishna M. From petunias to the present—a review of oligonucleotide therapy. J Clin Epigenet. 2017;3:38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mokri, N., Sepehri, Z., Faninam, F. et al. Chitosan-coated Zn-metal-organic framework nanocomposites for effective targeted delivery of LNA-antisense miR-224 to colon tumor: in vitro studies. Gene Ther 29, 680–690 (2022). https://doi.org/10.1038/s41434-021-00265-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00265-7